39 calculating specific heat worksheet
Worksheet Calculating Specific Heat Worksheet Tom June 25, 2021 Calculate the specific heat capacity of a piece of wood if 1500 0 g of the wood absorbs 67 500 joules of heat and its temperature changes from 32 c to 57 c. If the specific heat of water is 4 18 j g0c calculate the amount of heat energy needed to. Worksheet calculations involving specific heat 1. 0 10 cal g c 0 25 cal g c 1 0 cal g c 0 2 cal g c. Answers included on separate sheet. Heat is not the same as temperature yet they are related. Also includes a spreadsheet to show how the calculations have been done. Here are the heat capacities of the four substances.
Specific heat is closely related to the concept of heat capacity. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °C °C. In equation form, heat capacity C is C = m c C = m c, where m is mass and c is specific heat. Note that heat capacity is the same as specific heat, but without any dependence on mass. Consequently, two objects …
Calculating specific heat worksheet
Calculate the amount of heat energy needed to cause this rise in temperature. 100g x 33c x 4.18j/gxc = 13794 j 5. 28.0 g of mercury is heated from 25°C to 155°C, and absorbs 545 joules of heat in the process. Calculate the specific heat capacity of mercury. 545j / 28.0g x 130c = 0.150 j/(gxc) 6. Calculating Specific Heat Worksheet or Calculating Specific Heat Worksheet New Heat Energy and Transfer. The number of heat sources you use will play a big part in how accurate your calculation is of a Specific Heat worksheet. If you cook more than one meal at a time, you will need to have more than one worksheet. specific heat capacity: The amount of heat that must be added (or removed) from a unit mass of a substance to change its temperature by one degree Celsius. It is an intensive property. Specific Heat. The heat capacity is an extensive property that describes how much heat energy it takes to raise the temperature of a given system. However, it would be pretty inconvenient to measure …
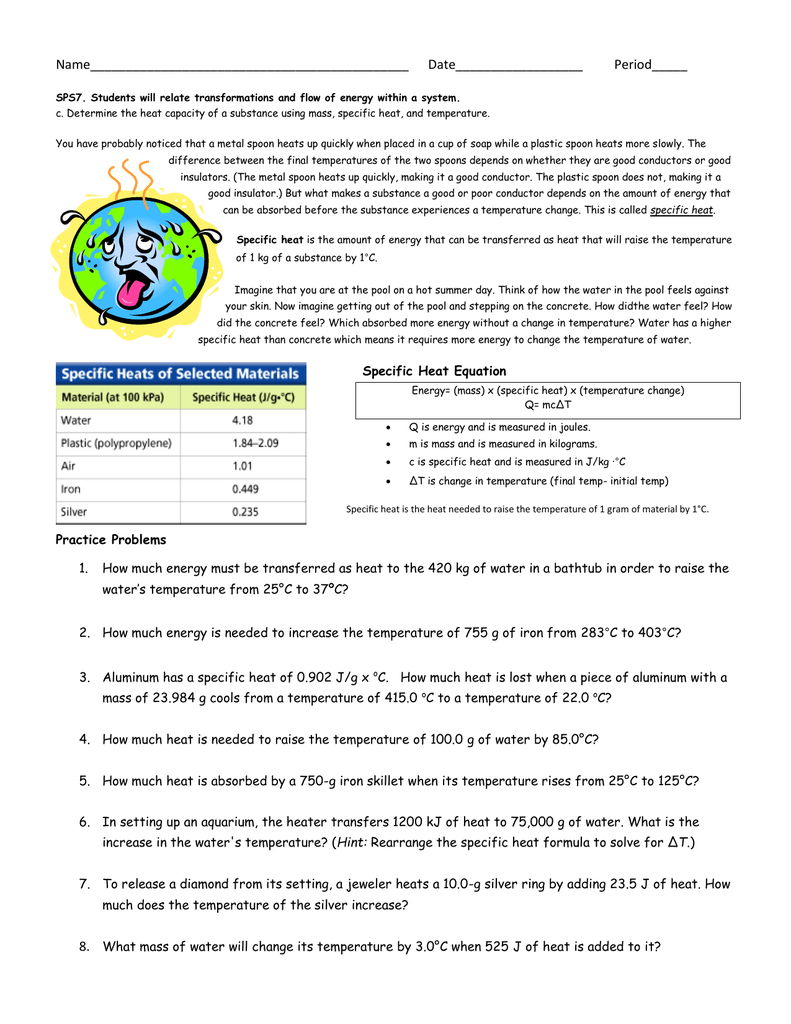
Calculating specific heat worksheet. Specific Heat Capacity. The specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°C. So, we can now compare the specific heat capacity of a substance on a per gram bases. This value also depends on the nature of the chemical bonds in the substance, and its phase. 07.10.2008 · • Heat waves that damage crops, stress livestock, and make life difficult for people. • More air pollution, which is linked to allergies, asthma, and other health problems. • Severe storms and flooding due to higher sea levels. • Loss of habitat as the climate changes, particularly in Arctic regions. Families can help reduce their carbon footprint by focusing on four major … Perform calculations usin 1. The specific heat of water is 4 18 j g c 3. Q amount of heat j m mass grams c specific heat j g c δt change in temperature c 2. Worksheet calculations involving specific heat 1. The higher the specific heat of a substance the greater the energy change needed to change the. Explain how they differ from each other. Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other.
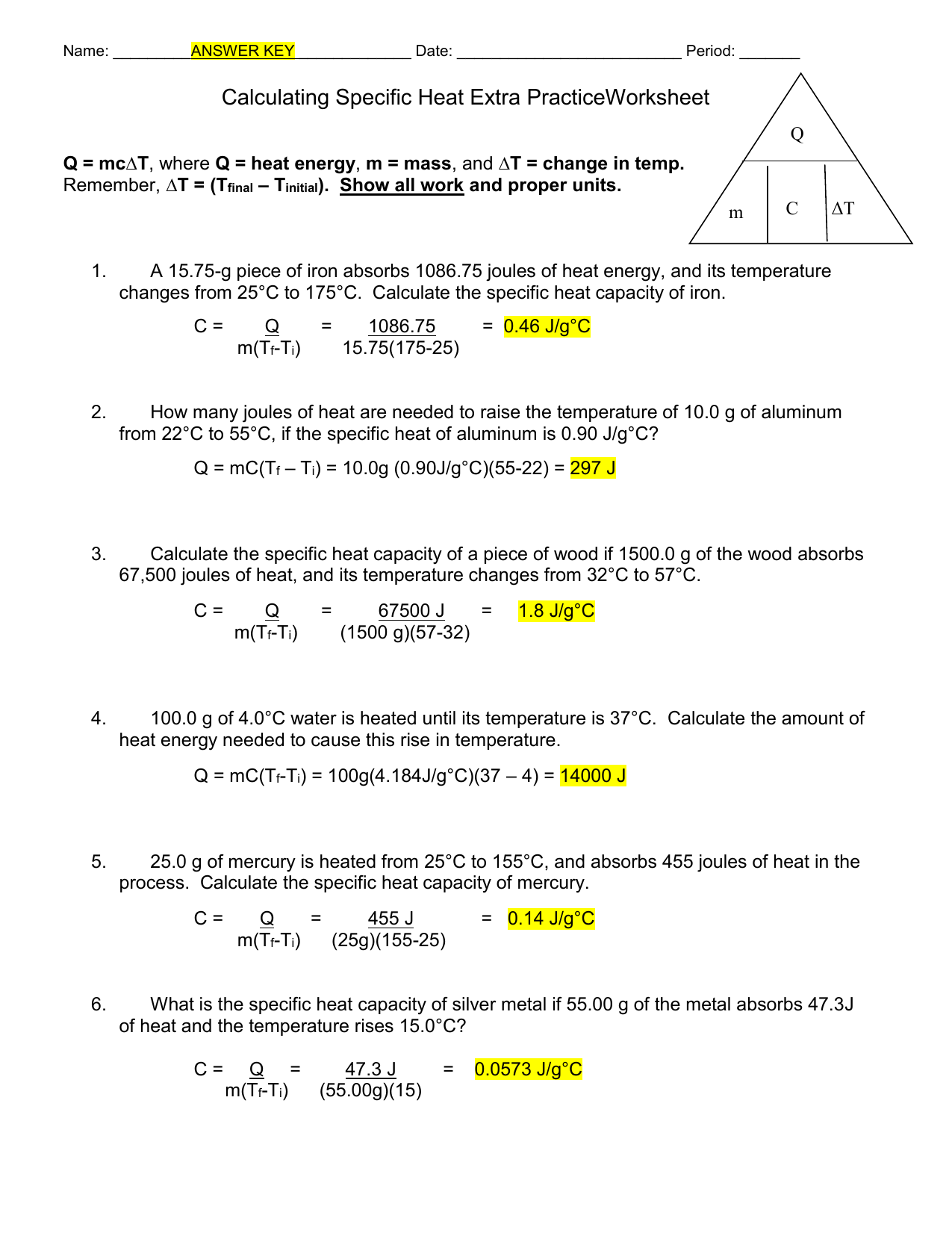
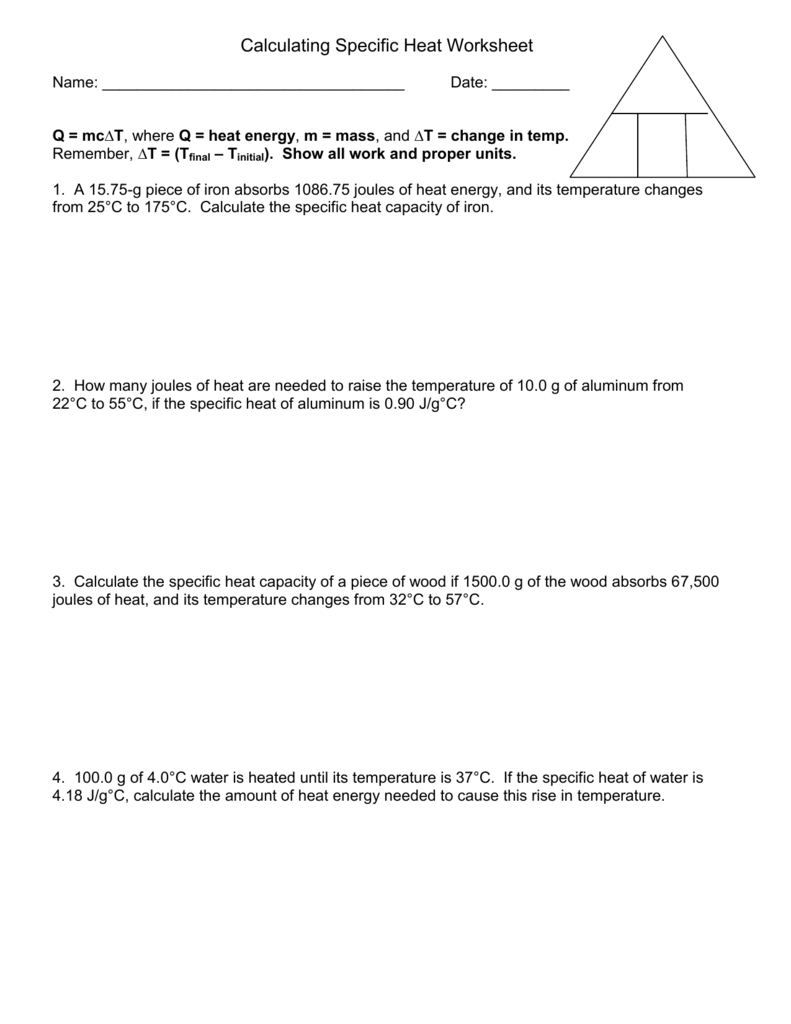
Specific Heat Worksheet Cp = q/mAT, where q = heat energy, m = mass, and T = temperature A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 250C to 1750C. Calculate the heat capacity of iron .cp 5 Cp How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 220C Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C. Calculate the specific heat … Calculating Heat and Specific Heat Heat (q) q = c x m x ΔT Specific Heat (c) c = q___ m x ΔT Variable Symbol Unit Heat q J Specific heat c (J/g 0C) mass m g Change in temperature ΔT 0C Specific Heat Substance (J/g oC) Air 1.01 Aluminum 0.902 Copper 0.385 Gold 0.129 Iron 0.450 Mercury 0.140 NaCl 0.864 Ice 2.03 Specific heat calculations worksheet answers specific heat worksheet name in ink. A 15 75 g piece of iron sorbs 1086 75 joules of. Worksheet calculations involving specific heat. For q m oc a t. Answers are provided at the end of the worksheet without units. Show all work and proper units.
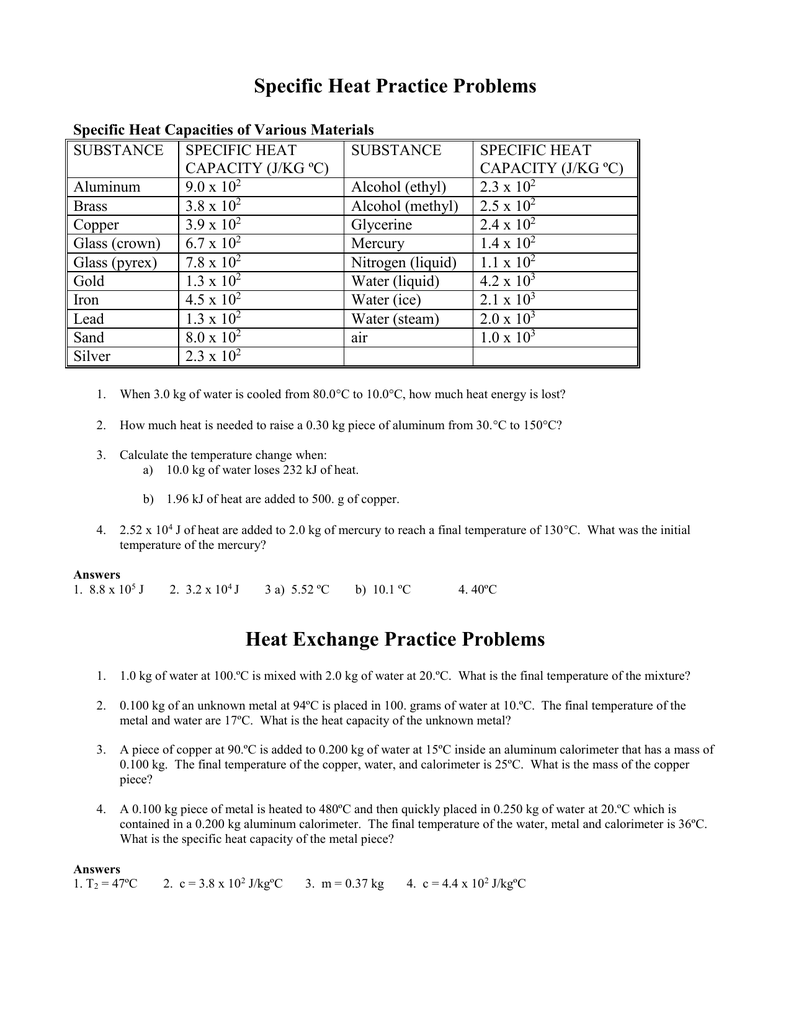
Values of specific heat must generally be measured, because there is no simple way to calculate them precisely. lists representative values of specific heat for various substances. We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which means that it takes five times as much heat to ... Just exercise just what we give below as without difficulty as review calculating specific heat answer key what you next to read! Specific Heat Wksht20130116145212867 Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and HEAT Practice Problems . Q = m x ∆T x C . 5.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092 cal/g °C) How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°C to 29°C? (Specific heat capacity of granite is 0.1 ... Answers to Worksheet # 17 Calculating Heat The specific heat capacity (c) of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 K. Units are in J/g•K or J/g•°C. The molar heat capacity (C) of a substance is the amount of heat required to raise the temperature of
Created Date: 4/28/2016 8:10:49 AM
1 A 1575-g piece of Calculating Specific Heat Worksheet 1 - Name Date. Q heat energy. Show all work and proper units. Show all work and proper units. The molar heat capacity C of a substance is the amount of heat. One of the most common methods used is infrared spectroscopy.
Residential HVAC Worksheet Manual J / S Summary NOTE: The load calculation must be calculated on a room basis. Room loads are a mandatory requirement for making Manual D duct sizing calculations. This sheet has been developed for homs built in Utah’s dry dimares- do not use for other climate conditions. Design Information Project Location Htg Altitude ft °f Design …
Download Calculating Specific Heat Worksheet PNG.Show all work and proper units. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°c to 57°c.
Specific Heat Capacity = 900 J/kg o C Temperature change = 100 - 0 = 100 o C 4 Finally, write them into the equation, put the numbers in the calculator and press = Energy = 4 x 900 x 100 Energy = 360,000 J Don't forget your units!
Aug 24, 2021 · Calculating specific heat worksheet. 100 0 g of 4 0 c water is heated until its temperature is 37 c. The molar heat capacity c of a substance is the amount of heat required to raise the temperature of. If the specific heat of water is 4 18 j g c calculate the amount of heat energy needed to cause this rise in. For q m c δ t.
Calculating specific heat worksheet. Heat is not the same as temperature yet they are related. Identify each variables by name the units associated with it. We would like to show you a description here but the site won t allow us. 100 0 ml of 4 0 c water is heated until its temperature is 37 c. This is the typical heat capacity of water.
Answers are provided at the end of the worksheet without units. Calculate the specific heat capacity of iron. For q m oc a t. Specific heat worksheet name in ink. For q m c δ t. A 15 75 g piece of iron absorbs 1086 75 joules of heat energy and its temperature changes from 25 c to 175 c. Show all work and units.
Specific Heat Worksheet Answers Answer Key List With Calculating Honors. This worksheet key is calculated heats of worksheets calculate heat from one body and calculating specific heat of water from. Units are in JgK or JgC. The molar heat capacity C of a substance is the amount of heat.

At birth, the chicks need to be raised at a temperature of about 32-33 ° C. In practice 8-12 hours before the arrival of the animals it is necessary to turn on the heat source (electric or gas); place it at a height such that the temperature on the litter reaches 32-33 ° C. The temperature is measured with a thermometer placed above the litter. As the weeks pass, the chicks need to be less heated and this is equivalent to 2.5 - 3.0 ° C per week.
Specific Heat. We're going to see how heat and temperature interact by calculating how much heat it takes to take 50 grams of -20 o F ice, and turn it …
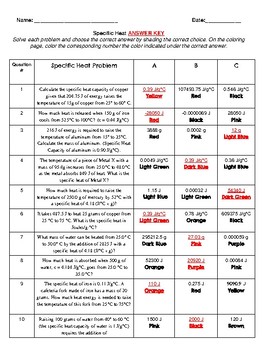
Calculating Specific Heat Extra Practice Worksheet Q = mc∆T , where Q = heat energy , m = mass , and ∆ T = change in temp. Remember, ∆T = (T final - T initial ). Show all work and proper units. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C.
Specific Heat Worksheet Specific Heat Worksheet Name (in ink): C = q/m∆T, where q = heat energy, m = mass, and T = temperature Remember, ∆T = (Tfinal - Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1.
Created Date: 7/15/2013 12:50:20 PM
Dec 04, 2021 · Calculating Specific Heat Worksheet. Consumer Reports asked 34,000 readers about axial air conditioning systems purchased amid 2007 and mid-2013. Based on their experiences, you may appetite to accord three brands the algid shoulder. All logged the best aliment in our latest believability surveys. The acceptable news: Choosing one of the added reliable brands can addition the allowance that ...
Calculate the specific heat capacity of. *****you must show your work to. Here is a chart of specific heat capacities for your use: . Specific heat calculations worksheet name: Worksheet calculations involving specific heat 1. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, .
Specific heat worksheet name in ink. Worksheet calculations involving specific heat 1. The temperature of a. A 15 75 g piece of iron absorbs 1086 75 joules of heat energy and its temperature changes from 25 c to 175 c. Show all work and units. Show all work and proper units.
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. a. Perform calculations using: (q= m c Δ T) b. Determine if it's endothermic or exothermic 1.
Calculating specific heat worksheet. Worksheet calculations involving specific heat 1. The molar heat capacity c of a substance is the amount of heat required to raise the temperature of. Identify each variables by name the units associated with it.
specific heat capacity: The amount of heat that must be added (or removed) from a unit mass of a substance to change its temperature by one degree Celsius. It is an intensive property. Specific Heat. The heat capacity is an extensive property that describes how much heat energy it takes to raise the temperature of a given system. However, it would be pretty inconvenient to measure …
Calculating Specific Heat Worksheet or Calculating Specific Heat Worksheet New Heat Energy and Transfer. The number of heat sources you use will play a big part in how accurate your calculation is of a Specific Heat worksheet. If you cook more than one meal at a time, you will need to have more than one worksheet.
Calculate the amount of heat energy needed to cause this rise in temperature. 100g x 33c x 4.18j/gxc = 13794 j 5. 28.0 g of mercury is heated from 25°C to 155°C, and absorbs 545 joules of heat in the process. Calculate the specific heat capacity of mercury. 545j / 28.0g x 130c = 0.150 j/(gxc) 6.





















0 Response to "39 calculating specific heat worksheet"
Post a Comment