39 chemistry average atomic mass worksheet answers
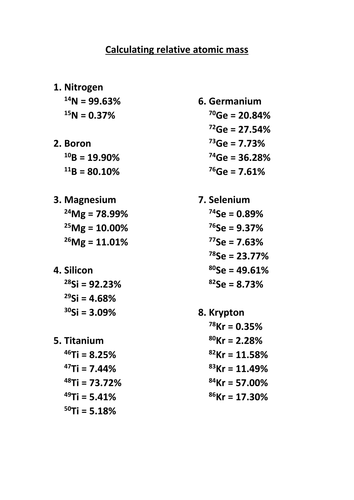
Chemistry Atomic Number And Mass Number Worksheet Answer ... What is the mass in grams of a one 1h atom. B one 12c atom. Some of the worksheets for this concept are chemistry work atomic number and mass number atomic numbers practice 1 chemistry average atomic mass work atomic structure work chapter 2 atoms and atomic molar mass work and key average atomic mass problems key 2013 atomic structure. 2.3: Calculating Atomic Masses (Problems) - Chemistry ... PROBLEM \(\PageIndex{4}\) Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments.
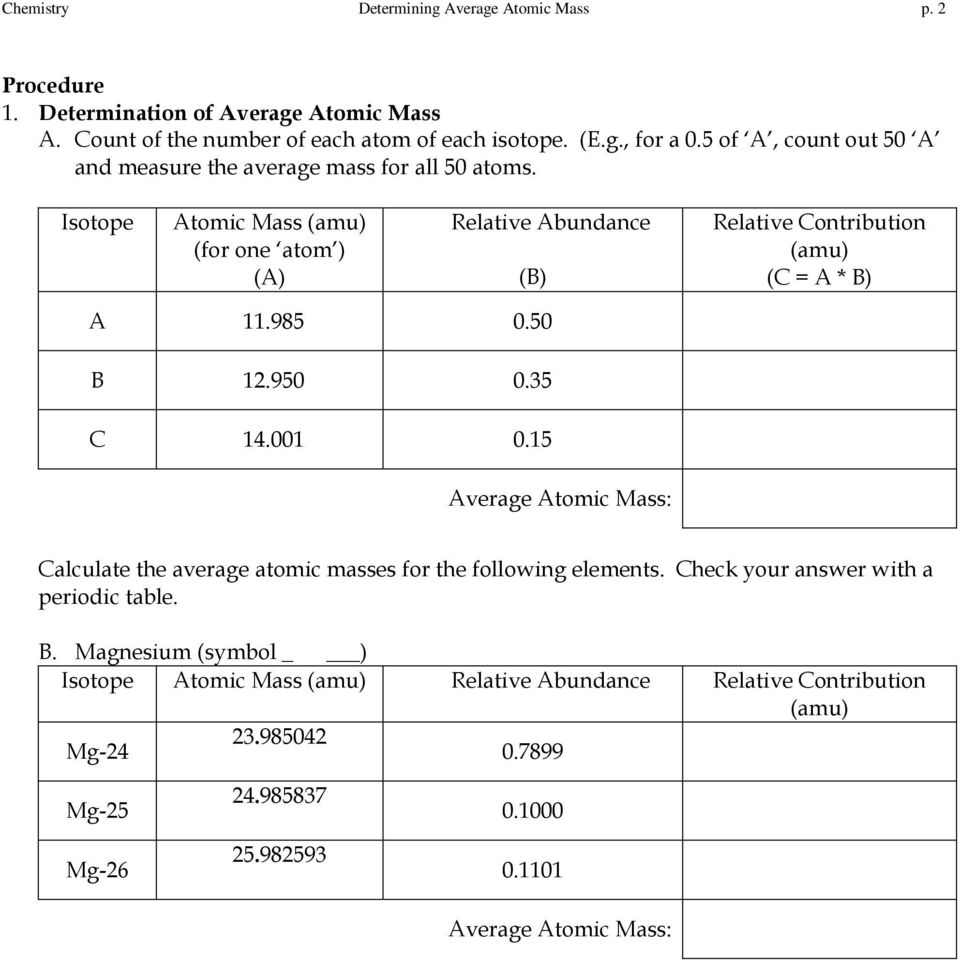
38 chemistry average atomic mass worksheet answers ... Basic atomic structure worksheet answers 1 a protons b neutrons c electrons a positive b neutral c negative 2 atomic number or identity. Pearson chemistry chapter 4. 15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below.
Chemistry average atomic mass worksheet answers
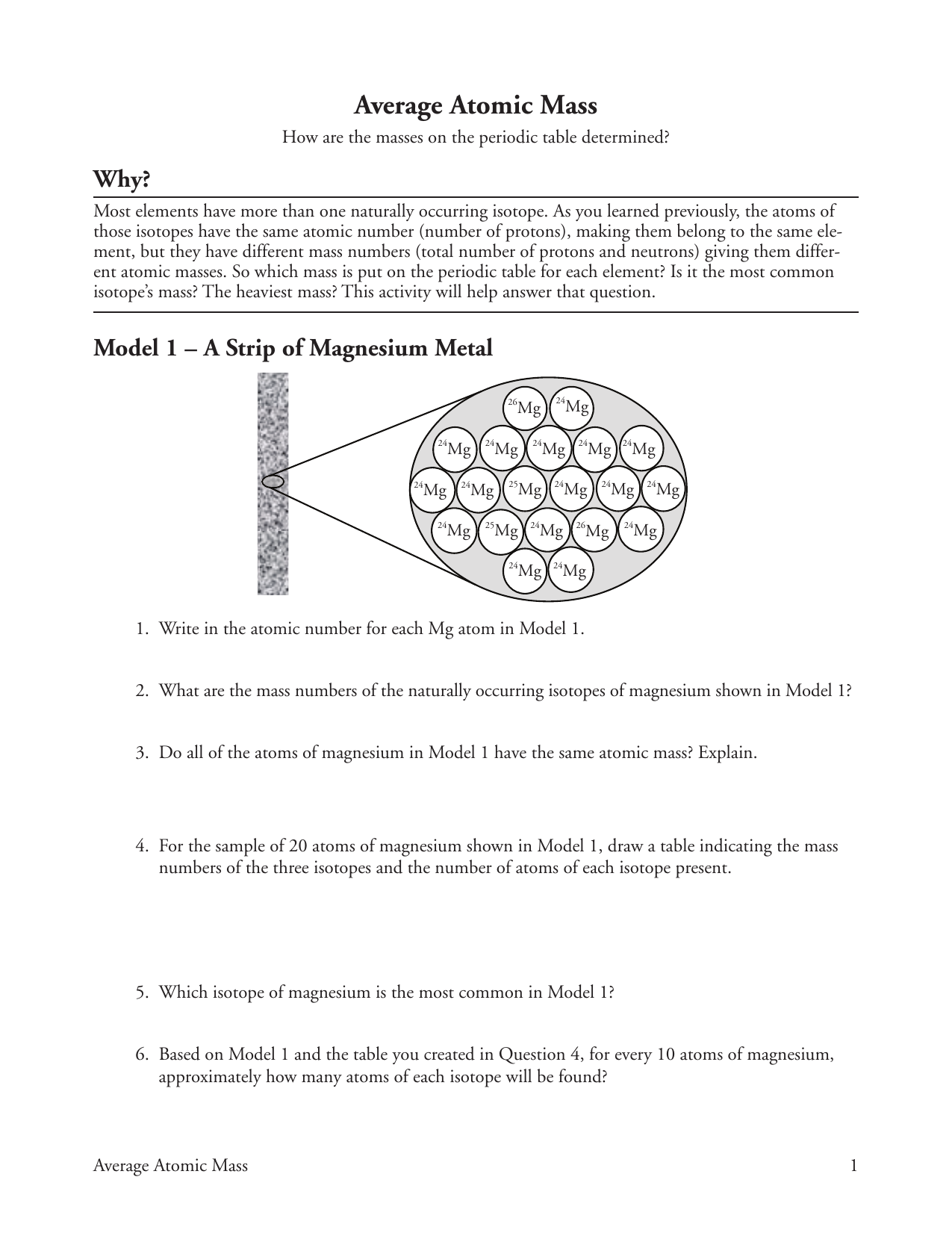
DOC Chemistry Worksheet - Forestville Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I. 3.1 Formula Mass and the Mole Concept – Chemistry The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. Experimental … PDF Answers Key for Unit Worksheets - Livingston The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu.
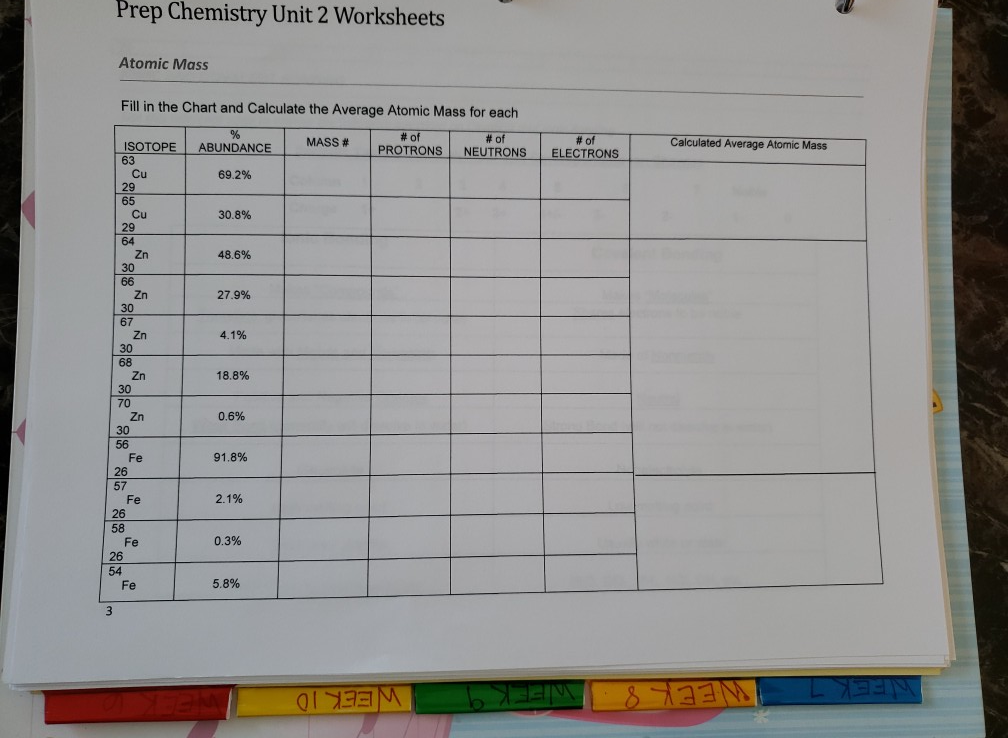
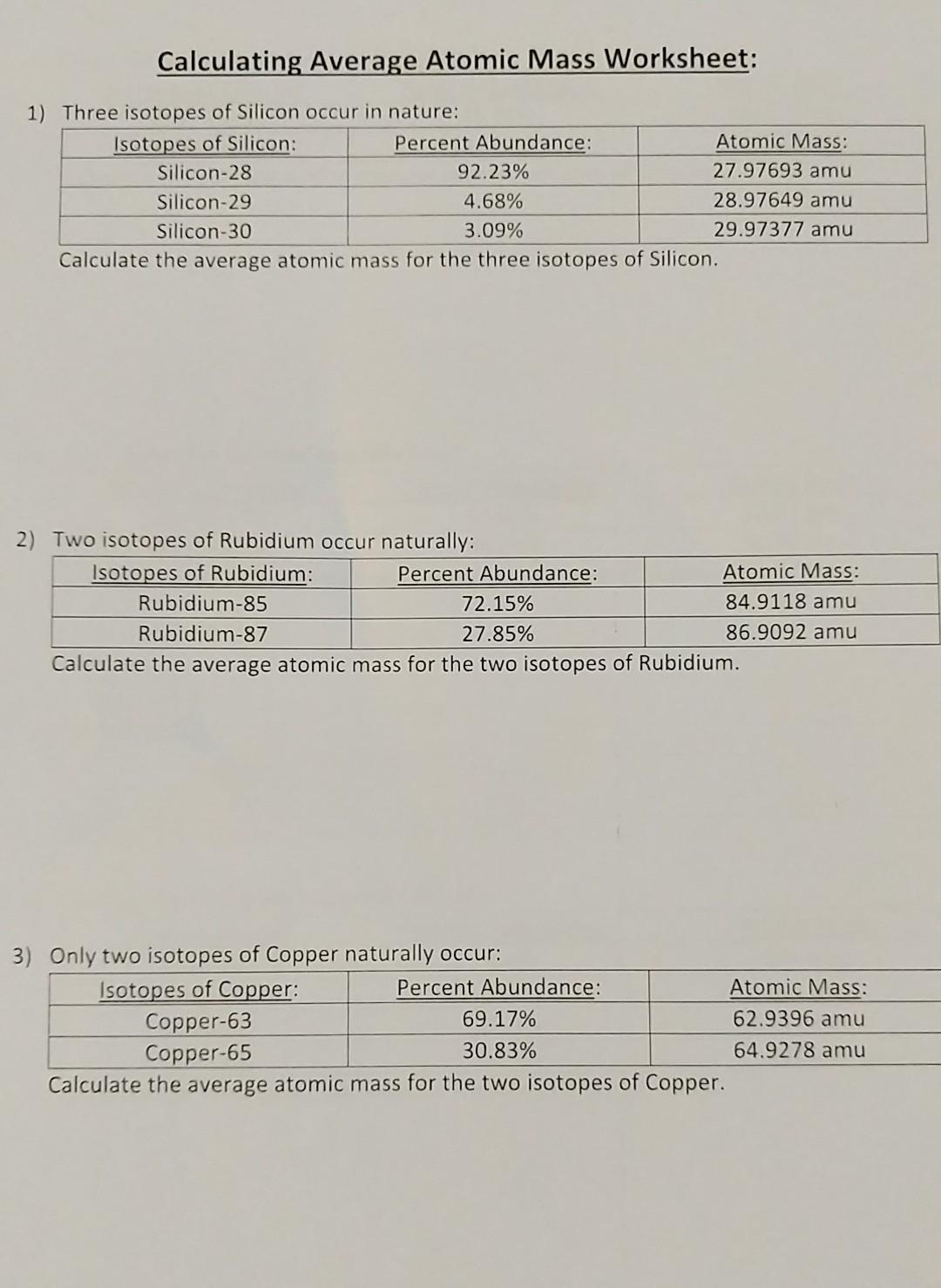
Chemistry average atomic mass worksheet answers. Chemistry Atomic Number And Mass Number Worksheet Answer ... Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium li 3 7 3 4 3 oxygen o 8 16 8 8 8. Complete the following chart and answer the questions below. Average Atomic Mass Worksheet Answers - worksheet Average atomic mass worksheet answers similar triangles worksheet. The estimating worksheet is designed to direct you. Silicon 30 3 09 29 97377 amu. Calculate the average atomic mass of this element. 1 three isotopes of silicon occur in nature. 90 92 abundance with 19 99 amu 0 26 abundance with 20 99 amu and 8 82 abundance with 21 99 amu. In ... Isotopes Worksheet Answers Chemistry - Worksheet Smart Isotopes worksheet answers chemistry. What is an isotope. êsym name 24 mg 30 108pd 13 35 s2 112 cd2 52 2 24 of atom or atomic mass of 53 94 56 no 30 125 69 80 ion. No carbon 12 and carbon 13 are isotopes of carbon but have different mass numbers. Naturally occurring isotope stable isotope radioactive isotope once you find your worksheet s you ... Average Atomic Mass Worksheet Answers Feb 25, 2022 · To witness this problem, attempt to create a worksheet named History. [newline]Excel doesn’t allow you to as a outcome of it makes use of the History worksheet as part of its change monitoring options (Section 23.3). Stunning Average Atomic Mass Worksheet Answers. Gorgeous Average Atomic Mass Worksheet Answers
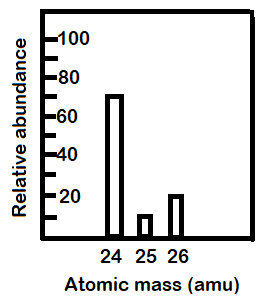
PDF KMBT 654-20131024112244 - Berger's Chemistry Class The average atomic mass is the weighted average of all the isotopes of an element. Example: A sample of cesium is 75% 133Cs, 20% 132Cs and 5% 134Cs. What is its average atomic mass? Answer: .75 x 133 = 99.75 .20 x 132 = 26.4 .05 x 134 = Total = 132.85 amu = average atomic mass Determine the average atomic mass of the following mixtures of ... Example of Molar Mass Calculation - ThoughtCo 2019-07-08 · Find the atomic mass for each element by using the mass given in the Periodic Table or table of atomic weights. Multiply the subscript (number of atoms) times the atomic mass of that element and add the masses of all of the elements in the molecule to get the molecular mass. Molar mass usually is expressed in grams (g) or kilograms (kg). PDF NAME Average Atomic Mass Worksheet: show all work. Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. Average Atomic Mass Worksheet Answers - Diy Color Burst Dec 12, 2021 · Calculate the average atomic mass. 24mg 7870 25mg 1013 and 26mg 117. If the atomic mass of 25Mg is 2498584 amu and 26Mg is 2598259 amu calculate the actual atomic mass of 24Mg. Average atomic mass gizmo worksheet answer key. Round all answers to two decimal places. 63 Cu has an atomic mass of 629298 amu and an abundance of 6909.
PDF WS-average atomic mass - Newton South High School Chemistry Worksheet NAME: _____ Natural Abundance and Average Atomic Mass Block: _____ 3. Naturally occurring chlorine has an average atomic mass of 35.453 amu. Chlorine exists in essentially two forms, Cl-35 and Cl-37. What is the natural abundance (mole percentage) of Cl-37 in a sample of chlorine? Isotope Worksheet Answer Key - ISD 622 Isotope Practice Worksheet Name: 1. 2. D. 4. 13 12 Here are three isotopes of an element: a. The element is: b. The number 6 refers to the ... Determine the average atomic mass of the following mixtures of isotopes. 128 127 126 a. t, 17%- 3% I (sðf' 8) 197 198 19 q. 5 55 56 Fe, 85% 55) 55.85 12 14 42 average atomic mass worksheet answer key - Worksheet ... Mar 03, 2022 · Average Atomic Mass Worksheet Answer Key Atomic Structure Worksheet. PDF Answers Key for Unit Worksheets - Livingston The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. Isotope Practice Worksheet - Sir Thomas Rich's School Isotope Practice Worksheet 1. Here are three isotopes of an element: 6 12C 6 13C 6 14C a. The element is: Carbon b. The number 6 refers to the atomic number c. The numbers 12, 13, and 14 refer to the mass number d. How many protons and neutrons are in the first isotope? 12 e. How many protons and neutrons are in the second isotope? 13 f. How many protons and neutrons …
40 chemistry atomic number and mass number worksheet answers Atomic Number And Mass Number Worksheet Answers Atomic Mass and atomic Number Worksheet Answers Along with Lovely atomic Structure Worksheet Unique 85 Best Chemistry atomic. It is important to remember that you will need to use this table for more than just learning about the periodic table.
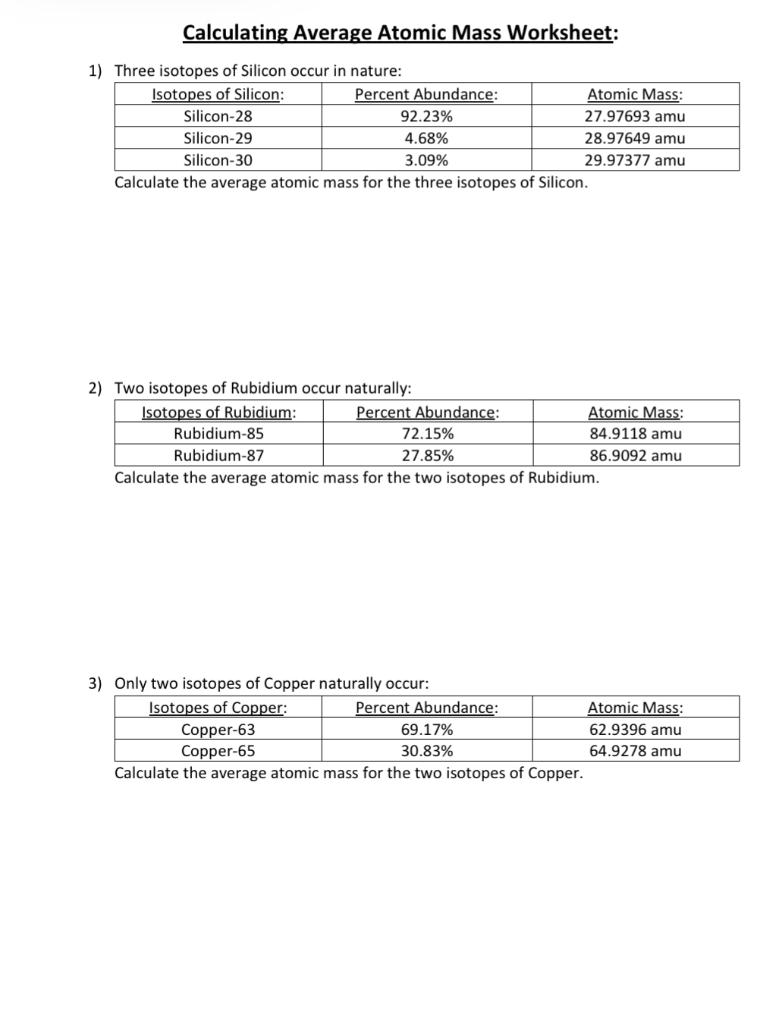
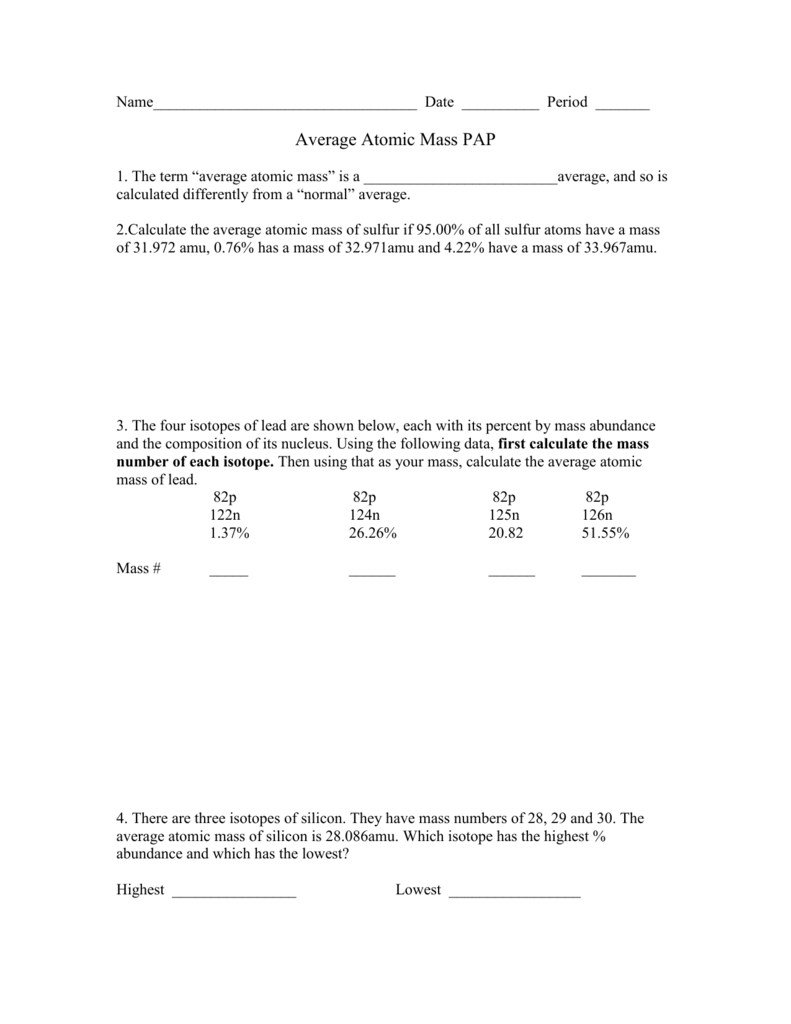
Average_Atomic_Mass_Worksheet.docx - Name Vitor Pires ... View Average_Atomic_Mass_Worksheet.docx from CHEMISTRY MISC at West Boca Raton High School. Name: Vitor Pires Period: Period 5 Average Atomic Mass Worksheet 1) Rubidium has two common isotopes, 85Rb
Answer Isotopes Mass And Key Worksheet Atomic [LJ29V6] Search: Isotopes And Atomic Mass Worksheet Answer Key
Chemistry Atomic Structure Worksheet Answer Key ... Chemistry is the study of matter its properties how and why substances combine or separate to form other substances and how substances interact with energy. Basic atomic structure worksheet answers 1 a protons b neutrons c electrons a positive b neutral c negative 2 atomic number or identity. Pearson chemistry chapter 4.
Chapter 1 Structure and Bonding - Chemistry Examination of carbon in periodic chart answers some of these questions. ... weighted average mass in atomic mass units (amu) of an element’s naturally occurring isotopes. Carbon: Atomic Number and Atomic Mass 12C 6 AC Z 13C 6 (98.9 12.000) (1.1 13.000) 12.011 100 × +× = Four different kinds of orbitals for electrons based on those derived for a hydrogen atom Denoted s, …
40 chemistry average atomic mass worksheet answers ... Jan 30, 2022 · 40 chemistry average atomic mass worksheet answers. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg (11.7%).
38 Isotopes And Average Atomic Mass Worksheet Answers ... Average Atomic Mass Worksheet Answer Key | PDF Wome Answer Ke Pd__ ate 'Accelerated Chemistry: Average Atomic Mass Worksheet Calculate the average atomic mass for each element based on the natural abundance of its isotopes. Atomic Mass Calculations "An atomic weight (relative atomic mass) of an element from a specified source is the ratio of ...
Chapter 6 – Quantities in Chemical Reactions – Chemistry As with atomic mass unit–based masses, to obtain the mass of 1 mol of a substance, we simply sum the masses of the individual atoms in the formula of that substance. The mass of 1 mol of a substance is referred to as its molar mass, whether the substance is an element, an ionic compound, or a covalent compound. Figure 6.2: The Amazing Mole. The major mole …
Molecular Mass Calculations - ThoughtCo 2019-03-11 · Formula or Molar Mass Worksheet Answers (pdf) ... This is because the periodic table lists values that are a weighted average of the mass of all natural isotopes of each element. If you are performing calculations using a molecule that contains a specific isotope, use its mass value. This will be the sum of the masses of its protons and neutrons. For example, if all the …
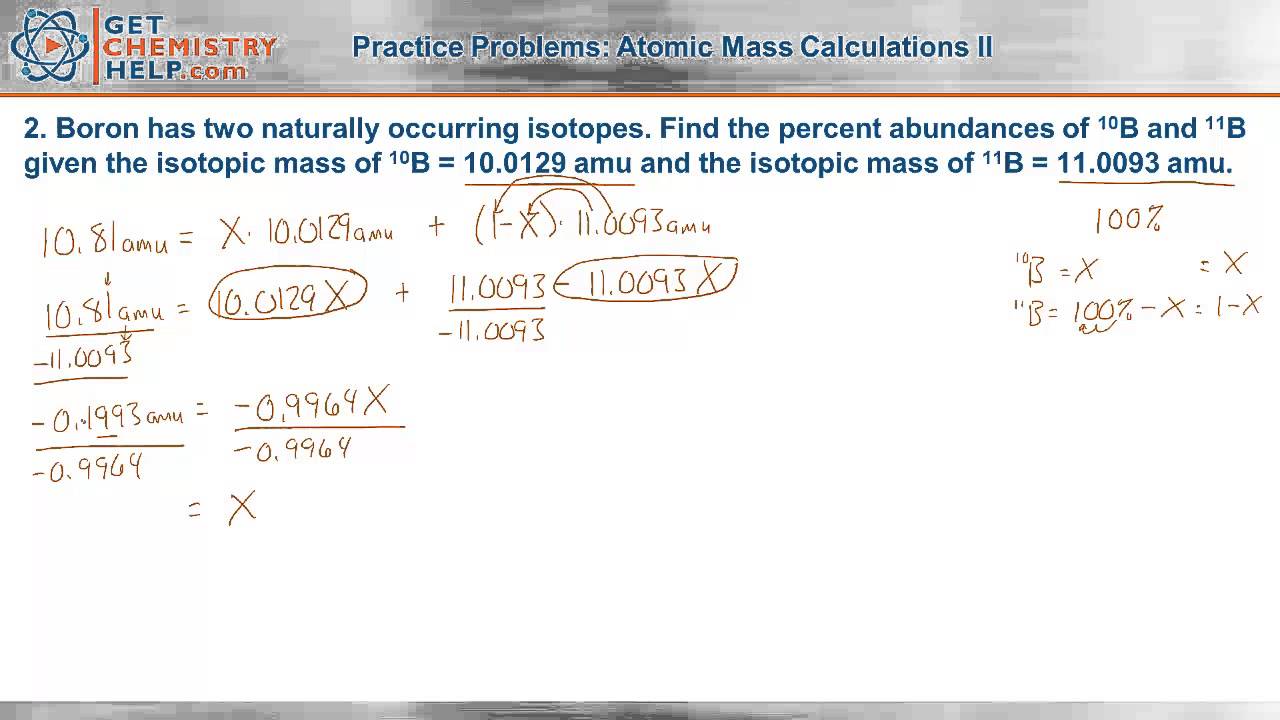
How to Calculate Average Atomic Mass | Chemistry | Study.com How to Calculate Average Atomic Mass: Example 1. Calculate the average atomic mass of boron given that 19.8% of its naturally occurring atoms have a mass of 10.013 amu and 80.2% have a mass of 11 ...
What is Atomic Mass? | Definition and Examples - Video ... 2021-08-15 · Here are three examples of atomic mass and average atomic mass: The atomic mass of most boron atoms is about 11 amu, because of their 5 protons and 6 neutrons. The average atomic mass of boron is ...
10th Grade Chemistry Atomic Number And Mass Number ... 10th grade chemistry atomic number and mass number worksheet answers. Atomic protons p n mass element. Some of the worksheets for this concept are chemistry work atomic number and mass number atomic numbers practice 1 chemistry average atomic mass work atomic structure work chemistry of matter chapter 2 atoms and atomic molar mass work and key ...
40 chemistry atomic number and mass number worksheet ... Chemistry atomic number and mass number worksheet answer key. 12 oxygen 8 8. Isotope atomic number z mass number a number of electrons 31p 15 18o 8 19 39 18 58ni2 58 2. Relative to the atomic symbol h c or mg where is the atomic number located in the isotope symbol. Refer to your periodic table. Atomic number and mass number.
Chemistry Worksheet Atomic Number And Mass Number ... Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53 74 53 uranium u 92 238 92 146 92 potassium k 19 39 19 20 19 lithium li 3 7 3 4 3 oxygen o 8 16 8 8 8.
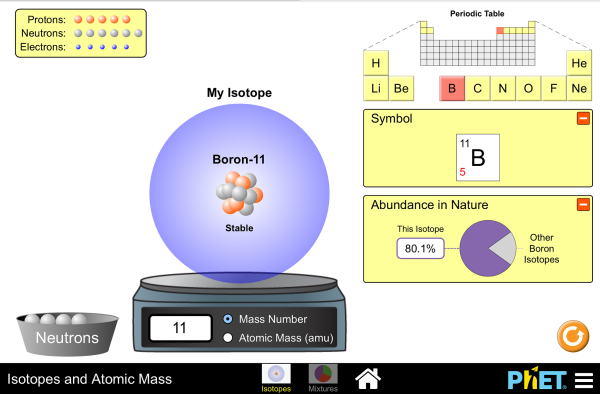
43 average atomic mass worksheet - Worksheet For You Phet Isotopes and Atomic Mass Worksheet Answers The atomic mass of an element is the average of its mass, so one atom will have a different mass than another. The periodic table does not ANSWER KEY BUILD AN ATOM PART I ATOM SCREEN Build an Atom from phet isotopes and atomic mass worksheet answers , source:docplayer.net.
40 calculating atomic mass worksheet answers - Worksheet ... How to Calculate Atomic Mass To calculate the atomic mass of a single atom of an element, add up the mass of protons and neutrons. Add these values together. The answer is the total atomic mass or atomic weight of the element. Example: You are given a sample containing 98% carbon-12 and 2% carbon-13. Calculating atomic mass worksheet answers.
10th Grade Chemistry Atomic Number And Mass Number ... Displaying top 8 worksheets found for atomic mass and atomic number answers. Calculate the average atomic mass for boron if it has two naturally occurring isotopes. Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 ...
Isotopes And Average Atomic Mass Chemistry Worksheet Answers ... Dec 21, 2021 · December 21, 2021. November 29, 2021. · Chemistry Worksheet. by David G. Fisher. Isotopes And Average Atomic Mass Chemistry Worksheet Answers – Worksheets are a low-cost, high-impact source. In a number of instances, these have actually been shown to be reliable in speeding up trainee learning. It is among the most commonly made use of ...
PDF Average Atomic Mass - Mr. Sault's Classroom 15. Use one of the methods in Model 3 that gave the correct answer for average atomic mass to calculate the average atomic mass for oxygen. Isotope information is provided below. Show all of your work and check your answer against the mass listed on the periodic table. Isotope Natural Abundance on Earth (%) Atomic Mass (amu) 160 99.76 15.9949
PDF Answers Key for Unit Worksheets - Livingston The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. X — amð 7) Magnesium consists of three naturally occurring isotopes. The percent abundance of these isotopes is as follows: 24 Mg (78.70%), 25Mg (10.13%), and 26Mg(11.7%). 'The average atomic mass of the three isotopes is 24.3050 amu.
3.1 Formula Mass and the Mole Concept – Chemistry The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. The formula mass of a covalent compound is also called the molecular mass. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. Experimental …
DOC Chemistry Worksheet - Forestville Average Atomic Mass. Calculate the average atomic masses. Round all answers to two decimal places. What is the atomic mass of hafnium if, out of every 100 atoms, 5 have a mass of 176, 19 have a mass of 177, 27 have a mass of 178, 14 have a mass of 179, and 35 have a mass of 180.0? Iodine is 80% 127I, 17% 126I, and 3% 128I.






















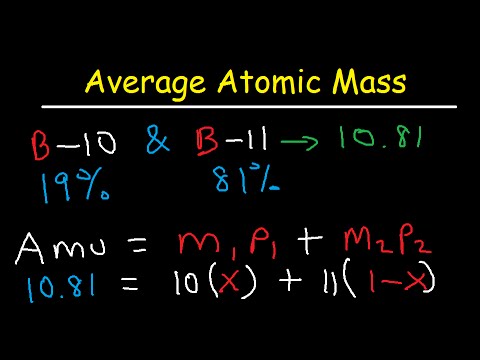

0 Response to "39 chemistry average atomic mass worksheet answers"
Post a Comment