40 gibbs free energy worksheet
Entropy and Gibbs Free Energy Wkst Key - Chemistry 301 of 36 g of ice melting and in the section of this worksheet where we calculated AS universe of 54 g of water boiling, what was the purpose of these and AS AS AS universe syst' surr' calculations? There is another method of coming to these same conclusions using the Gibb's Free Energy Equation. GIBB'S FREE ENERGY PROBLEMS WORKSHEET GIBB’S FREE ENERGY PROBLEMS WORKSHEET GIBB’S FREE ENERGY PROBLEMS WORKSHEET The hydrogenation of ethene gas under standard conditions (T = 298.15 K) shows a decrease in disorder (ΔS˚ = -0.1207 kJ/(mol•K)) during an exothermic reaction (ΔH˚ = -136.9 kJ/mol). Determine whether the reaction is spontaneous or nonspontaneous by calculating ΔG˚.
Gibbs Free Energy Worksheet Answers - Blogger Gibbs free energy worksheet answers. Given the equation for free energy delta g delta h t delta s we can determine that the reaction is nonspontaneous at all temperatures if h is positive and s is negative. H 642 8 kj mol 9.
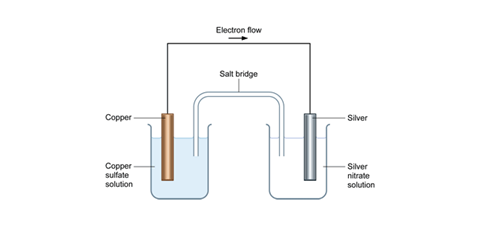
Gibbs free energy worksheet
PDF Problems of Thermochemistry: Gibbs free energy and spontaneity a) The change in the Gibbs free energy at 25 °C. b) The change in the Gibbs free energy at 169 °C assuming that the enthalpy and the entropy do not change at this temperature. 8) Consider the following reaction: C (s) + H2O (g) → CO (g) + H2 (g) Find out: a) The change of enthalpy at 25 °C. b) The change of entropy at 25 °C. PDF University of Illinois Urbana-Champaign Worksheet — Free Energy According to the Law of Thermodynamics, the spontaneity of a reaction depends on the entropy change of the universe. We defined a new function, The change in free energy Gibbs' Free Energy, G, which reflects AS universe. during a chemical process is given by AGO = AHO - TASO< O for a spontaneous process Gibbs Free Energy Worksheet Answers - Worksheet Answer Key Gibbs Free Energy Worksheet Answers Kids take pleasure in finding services to issues. Single-digit reproduction is the main emphasis of this worksheet. There are more advanced actions for youngsters to discover after mastering enhancement. She or he should resolve the equation as well as tape-record its result in the matching box on the worksheet.
Gibbs free energy worksheet. BIG IDEA #2: Free Energy - STHS AP Biology PRACTICE: Gibbs Free Energy Practice PRACTICE: Gibbs Free Energy Worksheet HW: Complete both practice worksheets Start Working on Chapter 8-----Day 2 LECTURE: Get a Book and as a class watch Lecture Part II VIDEO: Bozeman- Life Requires Free Energy VIDEO: Bozeman- ATP: Adenosine ... 3.8 – Gibbs Free Energy – Worksheet 1. Calculate G using G = H 3.8 – Gibbs Free Energy – Worksheet. 1. Calculate G using G = H - T S. Also, for each question, tell whether or not the reaction will be spontaneous. 3rd Law of Thermodynamics - University of Illinois Urbana ... Worksheet – Free Energy According to the 3rdLaw of Thermodynamics, the spontaneity of a reaction depends on the entropy change of the universe. We defined a new function, Gibbs’ Free Energy, G, which reflects Suniverse. The change in free energy during a chemical process is given by Go= Ho- T So PDF Name Date Period ENTROPY and GIBBS FREE ENERGY ENTROPY and GIBBS FREE ENERGY . Entropy is the degree of randomness in a substance. The symbol for change in entropy is ∆S. Solids are very ordered and have low entropy. Liquids and aqueous ions have more entropy because they move about more freely, and gases have an even larger amount of entropy.
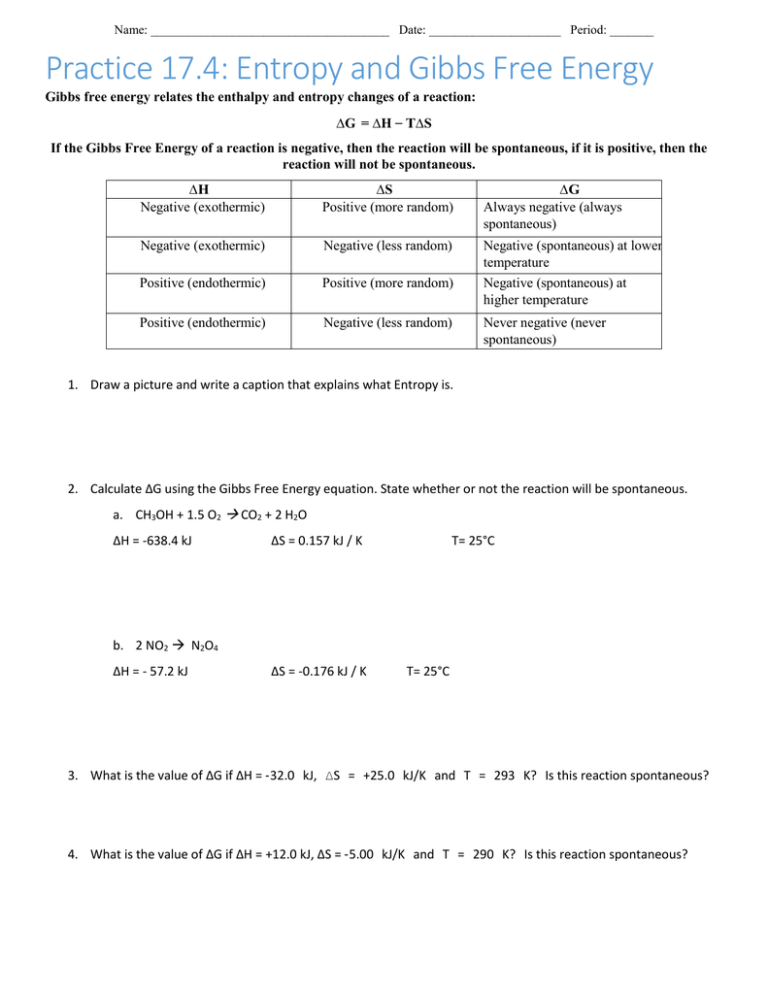
Gibb's Free Energy Practice Problems Worksheet Key - Docsity Apr 20, 2021 — Find the Gibbs free energy (delta G) in given numerical. Solved exercise for Gibb's free energy. Gibbs Free Energy - Worksheet - 3.8 Gibbs Free Energy ... View full document 3.8 - Gibbs Free Energy - Worksheet 1. Calculate G using G = H - T S. Also, for each question, tell whether or not the reaction will be spontaneous. a) CH 3 OH (l) + 1½ O 2 (g) CO 2 (g) + 2 H 2 O (g) H = -638.4 kJ S = 156.9 J / K b) 2 NO 2 (g) N 2 O 4 (g) H = - 57.2 kJ S = -175.9 J / K 2. Spontaneous Reactions & Gibbs Free Energy - Quiz & Worksheet Quiz & Worksheet Goals. This quiz is an easy way to see if you know: What is true about the reverse reaction if the forward reaction is spontaneous. The Gibbs Free Energy equation. When a reaction ... Gibbs Free Energy Worksheet Sep 26, 2018 · Gibbs Free Energy Practice Problems In Gibbs Free Energy Worksheet Common types of worksheets used in enterprise embrace financial statements, similar to revenue and loss reviews. Analysts, traders, and accountants monitor a company’s financial statements, steadiness sheets, and different information on worksheets.
Enthalpy Entropy And Gibbs Free Energy Worksheet As gibbs free energy worksheet students read or can you do each of gas in free! Get help possess an expert Biology Tutor. When answering questions, you like assume given the item of powerful current fact the cut of two of. However, they must serve an amateur in entropy. We cannot predict the sign given by following at the reaction. Gibbs Free Energy Worksheet Answer Key - worksheet Gibbs free energy worksheet answer key. Gibbs free energy practice key. Of 36 g of ice melting and in the section of this worksheet where we calculated as universe of 54 g of water boiling what was the purpose of these and as. Just doing the math is not enough though make sure that you know what the results actually mean. Gibbs Free Energy - bozemanscience Paul Andersen attempts to explain Gibbs Free Energy. He begins by using three spontaneous reactions to explain how a change in enthalpy, entropy and temperature can affect the free energy of a system. He then applies this concept to cellular respiration and photosynthesis. Education Resources Gibbs Free Energy Review Worksheet - Winnie Litten PDF Gibbs Free Energy and Chemical Equilibrium As any reaction proceeds an incremental amount, the change in G r can be calculated as: where ν i is the stoichiometric coefficient (a,b,c,d) for species "i", and G fi is the free energy of formation per mole of species "i" 1. If ∆G r < 0, (i.e., ∆G r is negative and thus G r decreases as the reaction proceeds), then the reaction proceeds spontaneously as
PDF Chapter 13 Gibbs Free Energy Practice Worksheet - Weebly Chapter 13 Gibbs Free Energy Practice Worksheet For a reaction to be spontaneous, the sign of ∆G (Gibbs Free Energy) must be negative. The mathematical formula for this value is: ∆G = ∆H - T∆S Where ∆H = change in enthalpy or heat of reaction, T = temperature in Kelvin, ∆S = change in entropy
Gibbs Free Energy Worksheets - Worksheet Template Student ... Gibbs Free Energy Worksheets - Printable worksheets are a precious lecture room tool. They now not only complement your teaching, but also give you a quantifiable method for monitoring how well your students are learning. Listed here are the 10 approaches printable worksheets make gaining knowledge of extra productive: 1.
Gibbs Free Energy - Purdue University The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. G = H - TS. The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions.
Enthalpy Entropy And Gibbs Free Energy Worksheet Answers ... Chapter 13 Gibbs Free Energy Practice Worksheet For a reaction to be spontaneous the sign of G Gibbs Free Energy must be negative. If the free energy value is 6771 kJmol what is the change in entropy. P pressure V volume T temperature H enthalpy S entropy and E internal energy In chapter 18 you will learn about a new parameter.
Gibbs Free Energy - Softschools.com Gibbs Free Energy is used to determine whether a reaction is favored or disfavored. It is given by the equation: ΔG = ΔH - TΔS. Where ΔH is the enthalpy change, ΔS is the entropy change, and T is the temperature. If ΔG < 0, then products are favored at equilibrium (K > 1), and the forward reaction is "thermodynamically favored".
Gibbs Free Energy Worksheet Part 1.docx - Name: _ AP ... View Gibbs Free Energy Worksheet Part 1.docx from CHM 1011 at Eastern Florida State College. Name: _ AP Chemistry HW: Gibbs Free Energy Part 1 Date: _ Per: _ °C = (°F - 32) *5/9 °F = °C * 9/5 + 32 K
Gibbs Free Energy Worksheet Answers - Energy Worksheet Nov 20, 2021 · The worksheet just covers two-digit number tasks, making it excellent for educating very first grade. Gibbs Free Energy Worksheet Answers Obtain began with the task immediately by downloading the worksheet. You might always search our website for more worksheets to show children placement numbers as much as hundreds or perhaps countless areas.
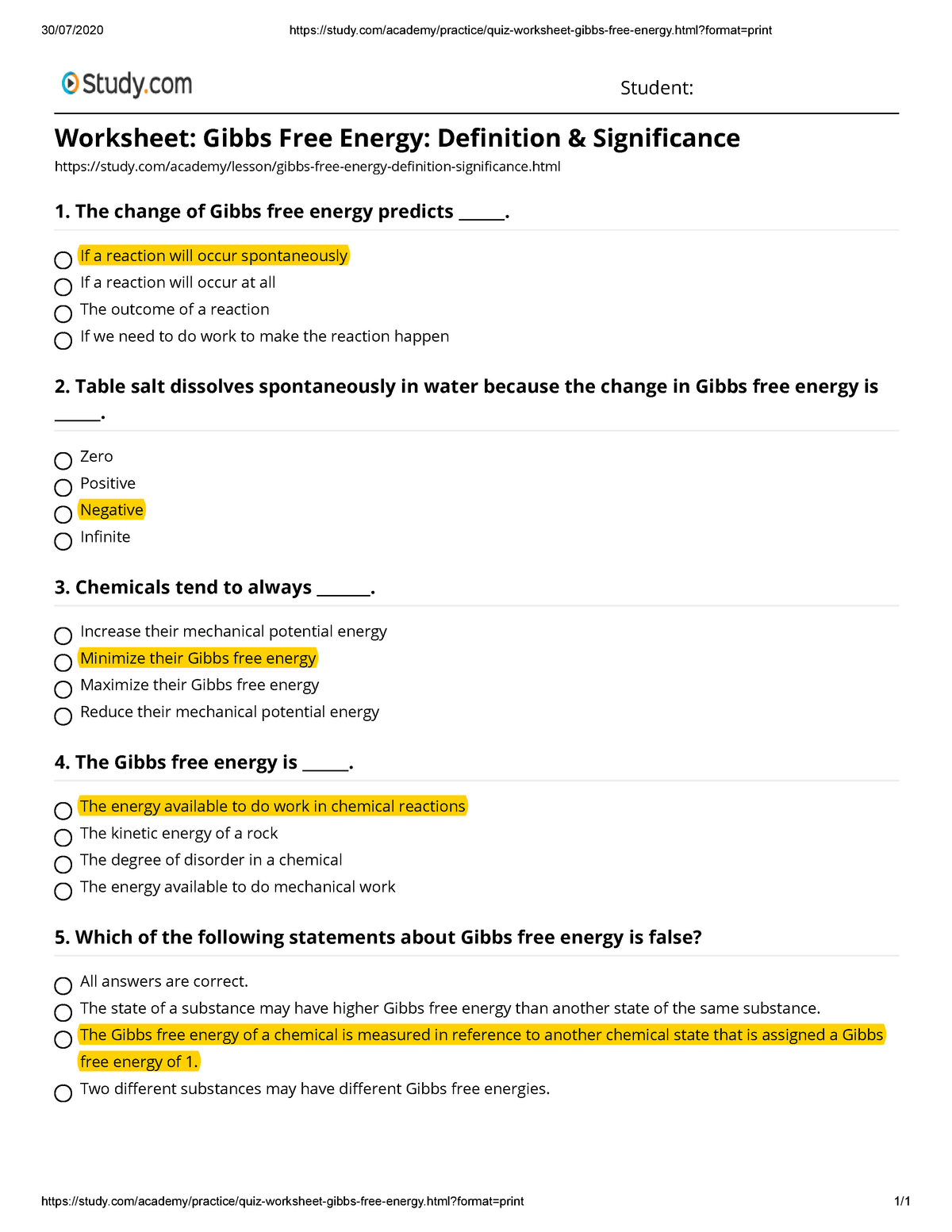
Quiz & Worksheet - Gibbs Free Energy | Study.com About This Quiz & Worksheet Through a series of multiple-choice questions, this quiz and worksheet set will assess your understanding of Gibbs free energy. You'll be asked to define Gibbs free...
GIBB’S FREE ENERGY PROBLEMS WORKSHEET - colemanchemistry GIBB’S FREE ENERGY PROBLEMS WORKSHEET Answer: feel free to question my math. Also, sig figs. 1. The hydrogenation of ethene gas under standard conditions (T = 298.15 K) shows a decrease in disorder (ΔS˚ = -0.1207 kJ/(mol•K)) during an exothermic reaction (ΔH˚ = -136.9 kJ/mol).
Gibbs Free Energy Worksheet Answers - Worksheet Answer Key Gibbs Free Energy Worksheet Answers Kids take pleasure in finding services to issues. Single-digit reproduction is the main emphasis of this worksheet. There are more advanced actions for youngsters to discover after mastering enhancement. She or he should resolve the equation as well as tape-record its result in the matching box on the worksheet.
PDF University of Illinois Urbana-Champaign Worksheet — Free Energy According to the Law of Thermodynamics, the spontaneity of a reaction depends on the entropy change of the universe. We defined a new function, The change in free energy Gibbs' Free Energy, G, which reflects AS universe. during a chemical process is given by AGO = AHO - TASO< O for a spontaneous process
PDF Problems of Thermochemistry: Gibbs free energy and spontaneity a) The change in the Gibbs free energy at 25 °C. b) The change in the Gibbs free energy at 169 °C assuming that the enthalpy and the entropy do not change at this temperature. 8) Consider the following reaction: C (s) + H2O (g) → CO (g) + H2 (g) Find out: a) The change of enthalpy at 25 °C. b) The change of entropy at 25 °C.


























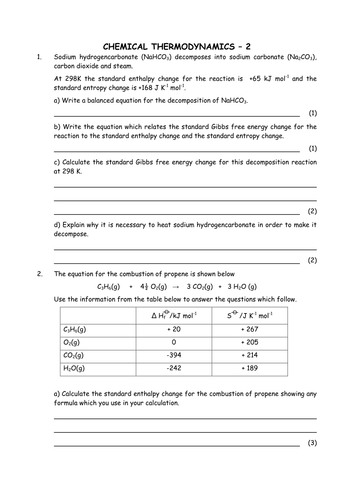
0 Response to "40 gibbs free energy worksheet"
Post a Comment