41 photoelectron spectroscopy worksheet answers
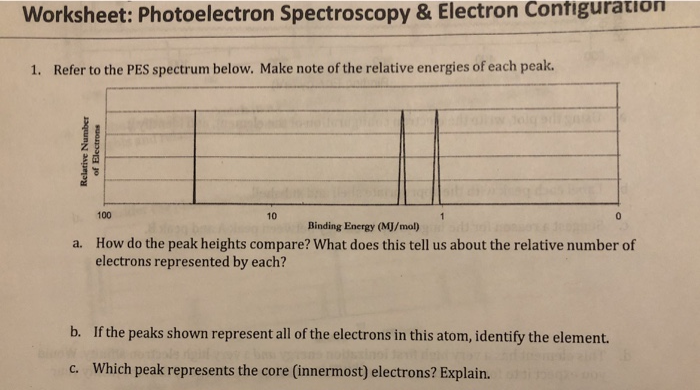
Solved Worksheet: Photoelectron Spectroscopy& Electron | Chegg.com Worksheet: Photoelectron Spectroscopy& Electron Contiguration 1. Refer to the PES spectrum below. Make note of the relative energies of each peak 100 Binding Energy (MJ/mol) How do the peak heights compare? What does this tell us about the relative number of electrons represented by each? a. b. PDF AP Chemistry Day 12 - Ms. Fleming the worksheet. - Discuss with your group if needed - Even if you think you received full points, think about how you could rewrite your answer to express your ideas clearly, concisely, and in a logical flow. Announcements • Unit Test ... Photoelectron Spectroscopy (PES) • Using PES, we can idenKfy specific elements and ...
Practice Quiz - Photoelectron Spectroscopy Photoelectron Spectroscopy. Click the "Start Quiz" button to proceed ...
Photoelectron spectroscopy worksheet answers
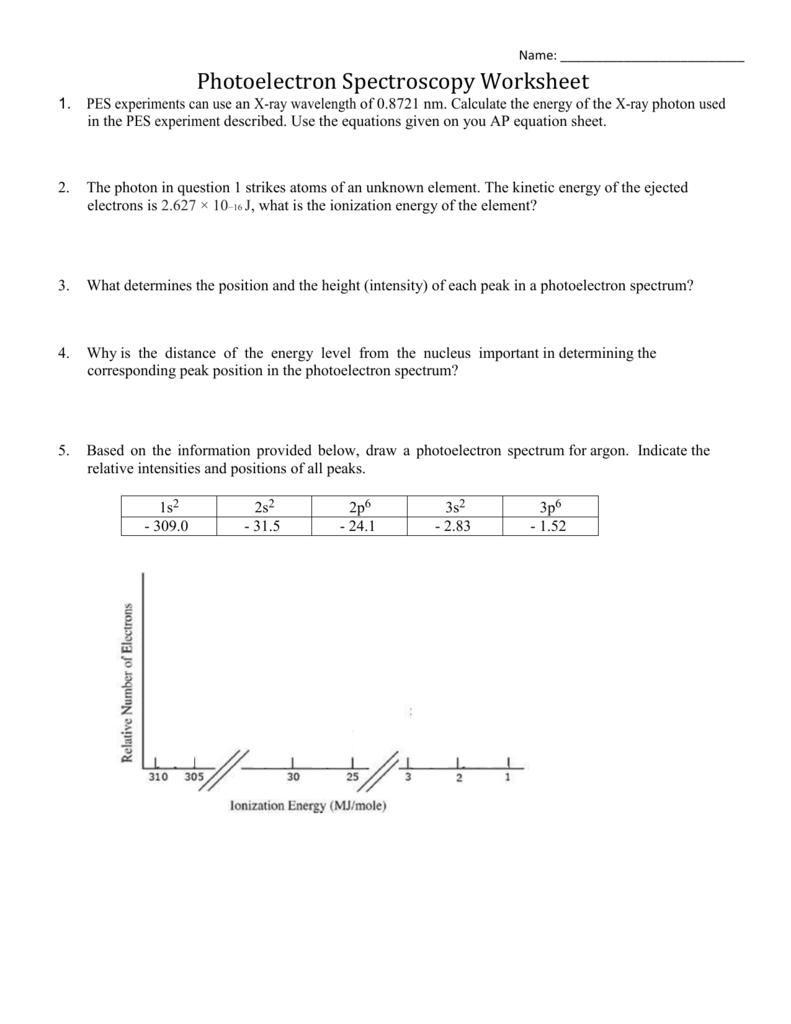
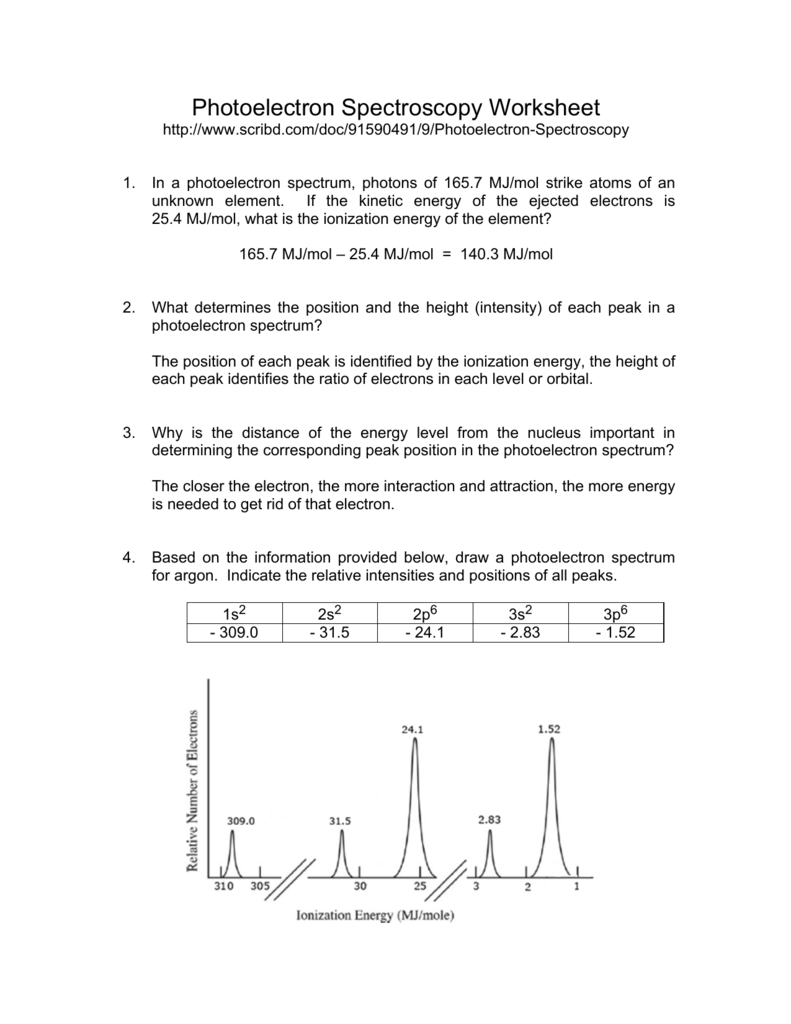
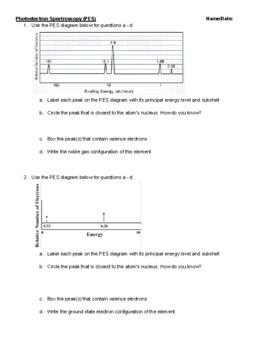
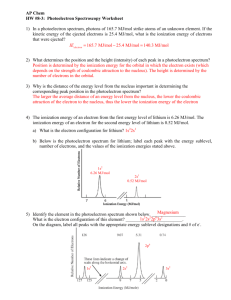
PDF Photoelectron Spectroscopy Worksheet - Ms. Ose's Chemistry Website 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2. 50 Photoelectron Spectroscopy Worksheet Answers - Pinterest Photoelectron Spectroscopy Worksheet Answers - 50 Photoelectron Spectroscopy Worksheet Answers , the Triple Point Teaching Resources F Fiveable 242 followers More information Photoelectron Spectroscopy Worksheet Answers Inspirational Ap Chemistry Big Idea 1 Electron Spectroscopy Pes Find this Pin and more on AP Chem by Fiveable. Science Teacher PDF Name: Date: AP Chemistry Big Idea 1 Worksheet: Photoelectron ... - Heroku 1. Refer to the PES spectrum below. Make note of the relative energies of each peak. a. How do the peak heights compare? What does this tell us about the relative number of electrons represented by each? b. If the peaks shown represent all of the electrons in this atom, identify the element. c. Which peak represents the core (innermost) electrons?
Photoelectron spectroscopy worksheet answers. Photoelectron Spectroscopy - Ultraviolet Photoelectron Spectroscopy ... Photoelectron spectroscopy (PES) is based on the photoelectric effect, the fact that matter irradiated by photons of sufficiently high energy emits electrons. Information about the sample is determined from the intensity, the angular distribution, and the spin of the emitted electrons. Photoelectron Spectroscopy - AP Chemistry - Varsity Tutors Possible Answers: Correct answer: Explanation: The binding energy at ~ 53 Mj represents the 1s orbital. With other peaks present, this 1s orbital is fully occupied by 2 electrons. The peaks at lower energy would correspond to the 2s and 2p, respectively. Since the 2p peak is twice the intensity of the 2s or 1 s peak, it has twice the electrons (4). PDF Photoelectron spectroscopy pogil- answers pdf - Silky Photoelectron spectroscopy pogil- answers pdf ... 3p 2 1s 2 2s 2 2p 6 3s 2 3p 1 1s 2 2s 2 2p 6 3s 2 Worksheet: Electron Configurations Name: More information Why? The chemical properties of an element are based on the number of electrons in the outer shell of its atoms. We use Lewis dot structures to map these valence electrons in order to ... Photoelectron Spectroscopy Worksheet Answers - Fallcitylodge.com Photoelectron Spectroscopy Worksheet Answers Posted by admin on August 20, 2022 It's a very simple, virtually four-step course of, however it can be a little time-consuming to get the essential info and put it into short, fast, and easy steps for each Partnership.
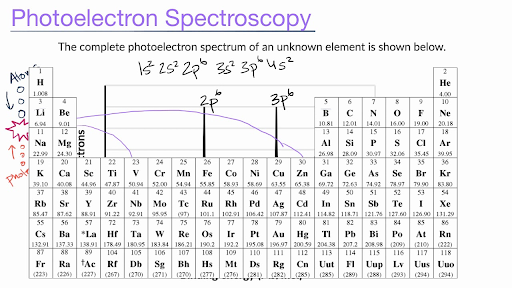
Handbook of X-Ray Photoelectron Spectroscopy 1995 33 Handbook of X-ray Photoelectron Spectroscopy Handbook of X-ray Photoelectron Spectrosco Carbon C Atomic Number 6 Is C as graphite C as graphite MonochromatedAlKa MgKa 1250 1200 Binding Energy (eV) KVV-----\ 1400 1200 1000 800 600 400 200 o 1200 1000 8(BindingEnergy (eV) Is ... Spectra Of Elements Worksheet Answers - Google Groups Answer announce the atoms of an element are excited by absorbing the energy from. Bohr's model does not accurately explain the chemical properties of elements. AP Chem HW 3 Photoelectron Spectroscopy Worksheet 1 In a photoelectron spectrum photons of 1657 MJmol strike atoms of an unknown element If the. MIT - Massachusetts Institute of Technology a aa aaa aaaa aaacn aaah aaai aaas aab aabb aac aacc aace aachen aacom aacs aacsb aad aadvantage aae aaf aafp aag aah aai aaj aal aalborg aalib aaliyah aall aalto aam ... Photoelectron_Spectroscopy_Worksheet-KEY.docx - Course Hero Photoelectron Spectroscopy (PES) ANSWER KEY 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? IE = Energy if Photons - KE of electrons = 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2.
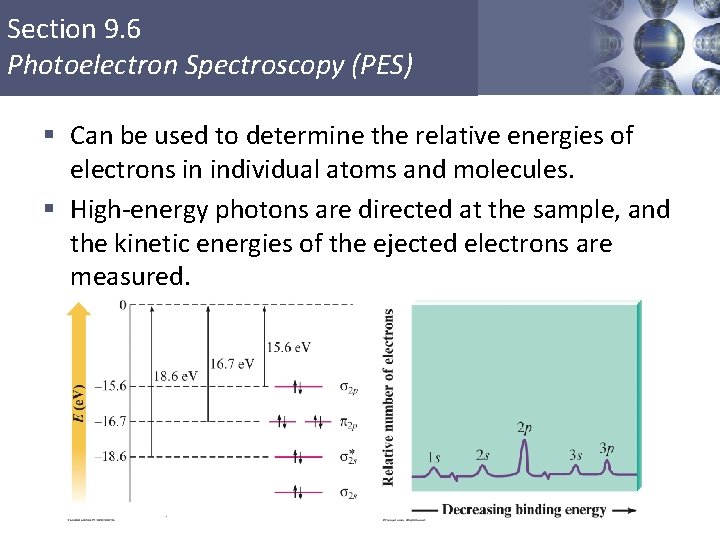
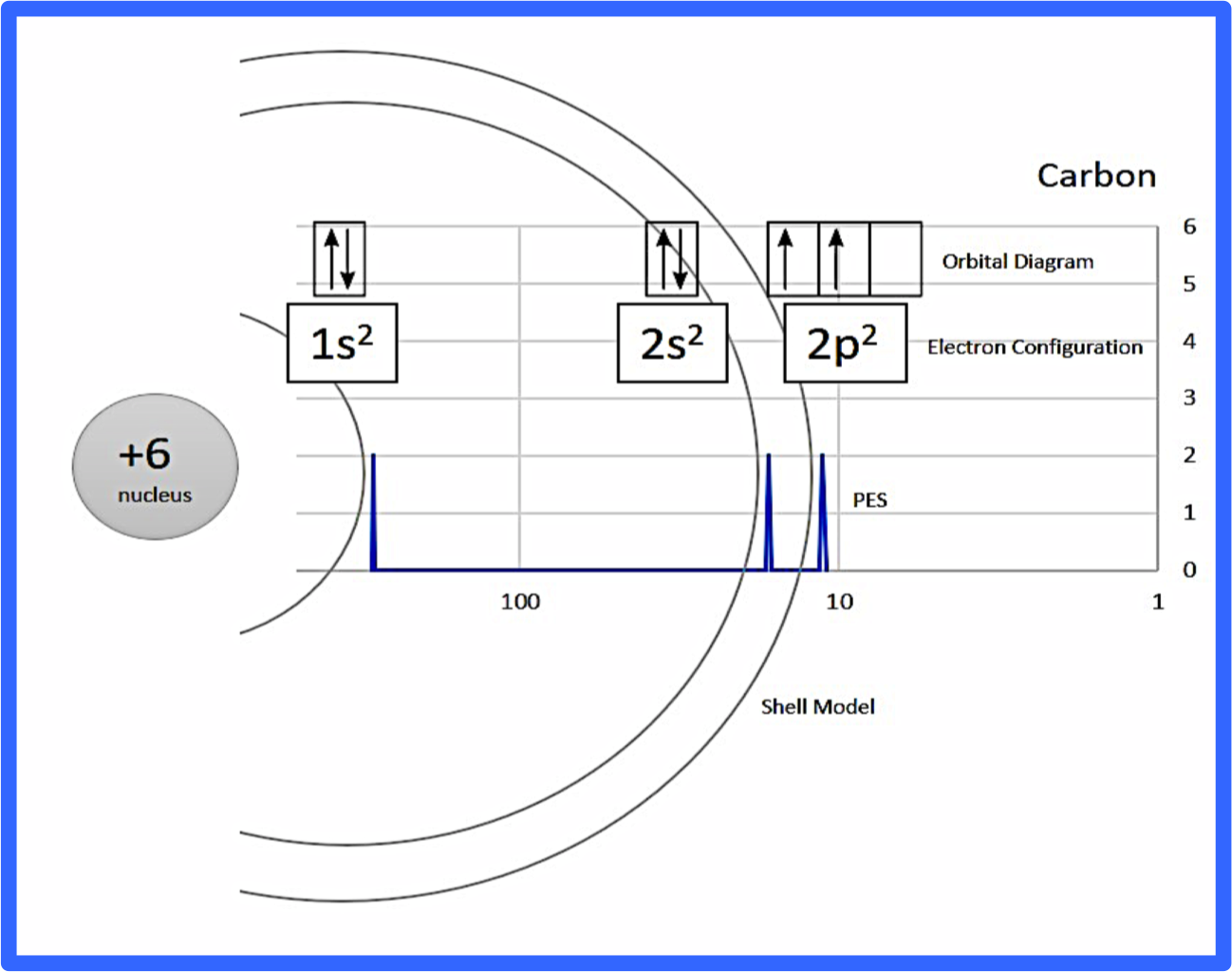
PDF Photoelectron Spectroscopy Worksheet - DePauw University Forn= 1 thereisasinglesubshell,whichwe identifyas1s;forn= 2 therearetwosubshells,whichweidentifyas2sandas2p. Thisallowsustowrite anelectronconfigurationthatdescribesanelement'selectrons. Giventhattheseelectronconfigurationfor lithiumis1s22s1,writetheelectronconfigurationforfluorine. Giventhedataavailabletoyou,howmany electronscanashelllabeledshold?p? Lifestyle | Daily Life | News | The Sydney Morning Herald The latest Lifestyle | Daily Life news, tips, opinion and advice from The Sydney Morning Herald covering life and relationships, beauty, fashion, health & wellbeing Photoelectron spectroscopy (article) | Khan Academy Practice: Photoelectron spectroscopy Science · AP®︎/College Chemistry · Atomic structure and properties · Photoelectron spectroscopy Photoelectron spectroscopy PDF 1.6 Photoelectron Spectroscopy AP Chemistry 1.5 Atomic Structure Day 5 ... Photoelectron spectroscopy (PES) allows scientists to determine the ionization energy of not only valence electrons, but all electrons in the atom. In PES, a gaseous sample of atoms is bombarded by X-rays or ultraviolet light (photons) of known energy. The kinetic energies of the photoelectrons that are ejected from the atoms are measured.
(PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu general-chemistry.pdf
NICI QID - Top 5 Modelle im Detail 4. Define the problem(s) the author has tried to solve. To See the unity of a book you need to know why it has the unity it has (supposing it’s a good book and it has a unity! ). To know why it has the unity it has you should know the authors main problem(s) he’s trying to answer; as well as subordinate questions and answers.
PDF Rancho High School 2. Refer to the PES spectrum below. 100 Binding Energy (M]/ mol) a. How many electrons are represented in each peak? b. How many electrons does the atom contain? How many electrons are in its valence shell? c. Write the electron configuration for this element. C)xqoevx 1.5 Photoelectron Spectroscopy & Electron Configuration 3.
Photosynthesis Worksheet Answer Key - Fallcitylodge.com Photoelectron Spectroscopy Worksheet Answers. It's a very simple, virtually four-step course of, however it can be a little time-consuming to get the essential info and put it into short, fast, and easy steps for each Partnership. Have you been looking for a Special Right Triangles Worksheet that can teach you tips on how to make it easier for ...
Photoelectron Spectroscopy, Spectrum, PES - AP Chemistry Worksheet Practice Unit 1.6: Photoelectron Spectroscopy (PES) Determining Binding Energy, Element's Name, & Electron Orbital, Electron Configuration, etc. Comparing Spectra of Two Atoms Justifying Differences in Peak Height and Binding Energy Over 20 PES Diagrams This worksheet is also available in Self-grading Google Form Organized Question Sets
Pogil Activities For Ap Chemistry Answer Key - myilibrary.org Pogil Activities For Ap Chemistry Answers Photoelectron. Details: 2 ™ Activities for AP* Chemistry POGIL 4. When a sample is injected into the mass spectrometer, do the atoms or molecules turn into positive or negative ions? Justify your answer with at least two pieces of evidence from Model 1. pogil photoelectron spectroscopy answer key.
PDF Pogil on Photoelectron Spectroscoy - Scarsdale Public Schools The actual photoelectron spectrum of neon is illustrated below: relative number of electrons 84 Ionization Energy (MJ/mol) a) Compare and contrast your predicted spectrum for neon with the above observed spectrum. b) Explain the significance of the size and position on the x-axis of the peak at 2 MJ/mol.
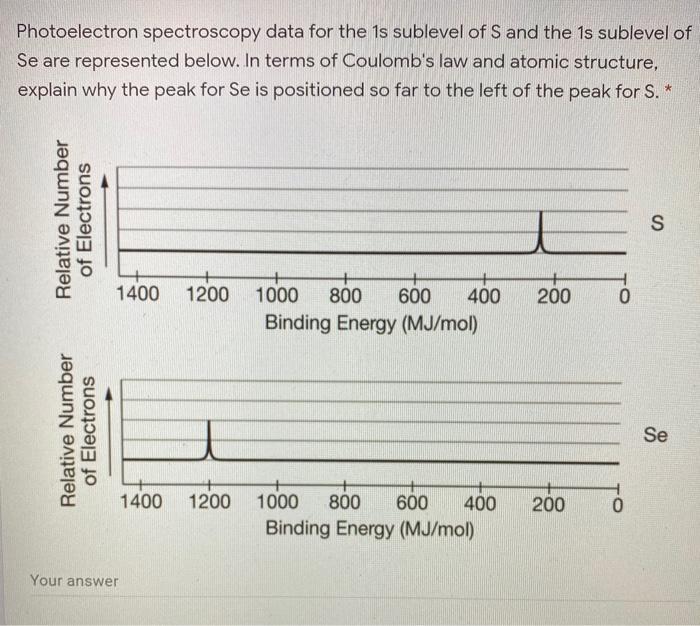
PDF 1.7 Periodic Trends 1.6 Photoelectron Spectroscopy AP Chemistry 1.5 ... Photoelectron Spectroscopy (you have the POGIL) kJ/mol now MJ/mol The minimum energy needed to remove an electron from an atom or ion in the gaseous state is defined as Ionization energy and the energy required to remove the least tightly held electron is the first ionization energy. Remember Coulomb's law F coulomb 𝝰 q 1 q 2 / r2.
Photoelectron Spectroscopy Pogil Answer Key Pdf Watch The Videos: The Electromagnetic Spectrum And How To ... 811; therefore, boron-11 is more abundant because the mass number is closer to the atomic mass. answers free download atomic spectra flinn chem topic lab answers''flame test and atomic spectra answer key april 25th, 2018 - flame test and atomic spectra answer key pdf free download here atomic spectra lab answer key answers on ...
Photoelectron Spectroscopy.docx - Leilani Salgado AP... Leilani Salgado AP Chemistry 9/27/20 Photoelectron Spectroscopy Questions 1. What determines the position and the height (intensity) of each peak in a photoelectron spectrum? The number of ejected electrons vs the corresponding ionization energy for the ejected electrons. Therefore, the peaks determine the electrons in different subshells.
PDF PHOTOELECTRON SPECTROSCOPY - pinchaschemsite.com 5. Below is shown the PES spectrum of sulfur (atomic number = 16). a. Write the full electron configuration of sulfur. b. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) c. On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13).
PDF Photoelectron Spectroscopy (PES) Worksheet #6 Name: Period: Seat# Explain here: Worksheet #6 Dougherty Valley HS Chemistry - AP Atomic Structure -Photoelectron Spectroscopy (PES) 4) Here is a PES spectrum of boron (Z=5) superimposed on that of fluorine (Z=9) a. Why are the fluorine peaks to the left of the boron peaks? b. Why is there one peak in fluorine that is so much taller than all the others?
Quiz & Worksheet - Photoelectron Spectroscopy | Study.com Instructions: Choose an answer and hit 'next'. You will receive your score and answers at the end. question 1 of 3 The 1s electrons for helium have a binding energy of 2.37 MJ/mol, while the 1s...
PDF AP WORKSHEET 01g: Photoelectron Spectroscopy (a) Write the electron configuration and identify the element. (2) (b) The plot is divided into three separate areas on the x-axis. Why is the axis divided in this manner? (2) (c) What would be the charge on an ion formed from this atom? Justify your answer. (2) (d) What is the significance of three of the peaks having the same height? (2)
Photoelectron Spectrum - An Overview of Photoelectron Spectrum and ... UPS and XPS are both photoelectron spectroscopy techniques, with UPS standing for ultraviolet photoelectron spectroscopy and XPS for X-ray photoelectron spectroscopy. In the near-surface region, XPS uses high-energy X-ray photons to excite "core" electrons, whereas UPS uses lower-energy photons in the deep UV range to excite valence electrons.
PDF Photoelectron Spectra Samples Photoelectron'Spectroscopy'(PES)Sample'Questions'! ' Questions'113refer'to'the'photoelectron'spectrum'of'neon'shown'below:' ' J. K. Howell R e l a t i v e N u m b e r o f E l e c t r o n s 1000 800 600 400 200 0 Photoelectron Spectrum of Neon Binding Energy (eV) A B C
PDF Name: Date: AP Chemistry Big Idea 1 Worksheet: Photoelectron ... - Heroku 1. Refer to the PES spectrum below. Make note of the relative energies of each peak. a. How do the peak heights compare? What does this tell us about the relative number of electrons represented by each? b. If the peaks shown represent all of the electrons in this atom, identify the element. c. Which peak represents the core (innermost) electrons?
50 Photoelectron Spectroscopy Worksheet Answers - Pinterest Photoelectron Spectroscopy Worksheet Answers - 50 Photoelectron Spectroscopy Worksheet Answers , the Triple Point Teaching Resources F Fiveable 242 followers More information Photoelectron Spectroscopy Worksheet Answers Inspirational Ap Chemistry Big Idea 1 Electron Spectroscopy Pes Find this Pin and more on AP Chem by Fiveable. Science Teacher
PDF Photoelectron Spectroscopy Worksheet - Ms. Ose's Chemistry Website 1. In a photoelectron spectrum, photons of 165.7 MJ/mol strike atoms of an unknown element. If the kinetic energy of the ejected electrons is 25.4 MJ/mol, what is the ionization energy of the element? 165.7 MJ/mol - 25.4 MJ/mol = 140.3 MJ/mol 2.




















0 Response to "41 photoelectron spectroscopy worksheet answers"
Post a Comment