45 2.3 elements and compounds worksheet answers pearson
K To 12 Science Grade 7 Learners Material - Module - Scribd With the 118 elements, imagine how many combinations of elements you can make into compounds and how diverse the materials around us can be. In Modules 4 and 5, you will learn more about the compounds and elements. You will work on different samples of compounds and elements and explore their properties. Compounds are made up of elements. PDF Chemistry 20 Workbook Chemistry 20 Worksheets 6 Worksheet 1.3: Reactions Complete the following reactions, identify the reaction type and balance the equation.(3 marks each; 15 marks total) 1) mercury (II) oxide is broken down into its elements by heating. 2) a nickel strip is placed in a gold (III) sulfate solution 3) phosphoric acid reacts with iron (III) oxide.
PDF 2.1 Properties of Matter 2 - Weebly describe elements and compounds. In this text, substance and pure substance are synonyms. A sample of matter can be described as pure if the amount of impurities in the sample is negligible. Download a worksheet on Physical Properties of Matter for students to complete, and find additional teacher support from NSTA SciLinks. L2 L1 Gifted and ...
2.3 elements and compounds worksheet answers pearson
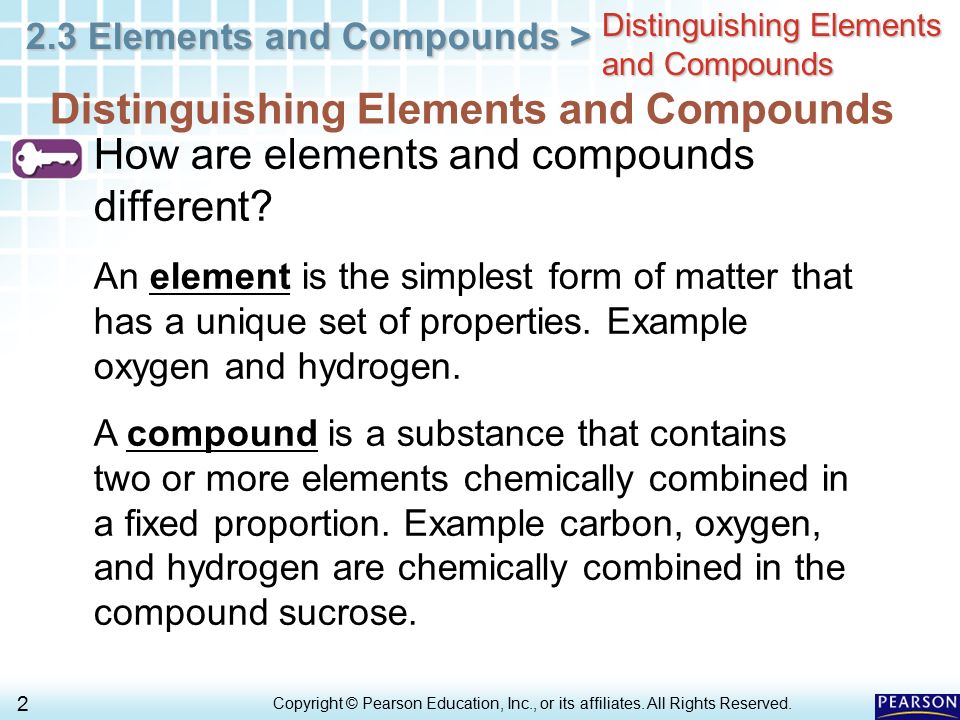
Chemistry 2.3: Elements and Compounds Flashcards | Quizlet element the simplest form of matter that has a unique set of properties. ex. oxygen, hydrogen compound a substance that contains two or more elements chemically combined in a fixed proportion. ex. sucrose is a compound made of carbon, oxygen, and hydrogen. has fixed composition (same chemical formula) chemical change PDF Chapter 2 The Chemical Basis of Life - Los Angeles Mission College 2.3 Elements can combine to form compounds Compound—a substance consisting of two or more different elements combined in a fixed ratio -There are many compounds that consist of only two elements - Table salt (sodium chloride or NaCl) is an example - Sodium is a metal, and chloride is a poisonous gas PDF Pearson Queensland Chemistry 11 Skills and Assessment Book WORKSHEET 2.2.3 Molarity—measuring moles in solution 162 WORKSHEET 2.2.4 Solving solubility—predicting precipitation reactions 163 WORKSHEET 2.2.5 Concentration and strength— picturing acids and bases 164 WORKSHEET 2.2.6Reactions of acids— predicting products 165 WORKSHEET 2.2.7 Literacy review—full and ionic chemical equations 166
2.3 elements and compounds worksheet answers pearson. Section 2.3 Elements And Compounds Flashcards | Quizlet The properties of compounds are different from those of their component elements. true. Sodium chloride is a _______ of sodium, which is a soft _______, and chlorine, which is a pale yellow ________. white solid, gray metal, green poisonous gas. Describe one way to decide whether a sample of matter is a substance or a mixture. Unit 2: Matter & Moles - Chemistry-2 Mr. Nordahl - Google Reading Guide 2.1-2.3. PowerPoint Elements/Compounds/ Mixtures. Pearson E-Text 2.4; Chemical changes and Conserv. of Mass. Matter Worksheet. Matter Worksheet Answers. Pearson Online Problem set. Pearson E-Text 16.1; Solubility. Solubility Worksheet. Pearson E-Text 10.1; The Mole. Kahoot! You need to enable JavaScript to run this app. Kahoot! You need to enable JavaScript to run this app. PDF Chapter 2 The Chemistry of Life Worksheets - Chandler Unified School ... Table2.1: Types of Organic Compounds TypeofCompound Examples Elements Functions Carbohydrates sugars,starches carbon,hydrogen,oxy-gen providesenergytocells, stores energy, forms bodystructures Lipids fats,oils carbon,hydrogen,oxy-gen stores energy, forms cellmembranes,carries messages Proteins enzymes,antibodies carbon,hydrogen,oxy-gen,nitrogen ...
pearson education worksheet answers math answer pearson maths 2.3 Elements And Compounds Worksheet Answer Key → Waltery Learning walthery.net prentice implied decimals 21 PEARSON EDUCATION MATH WORKSHEETS GRADE 5 PDF mathworksheetss.blogspot.com pearson glencoe mcgraw jugem factoring precalculus prentice inequalities workbook PDF 2.1 Properties of Matter 2 - Henry County Schools describe elements and compounds. In this text, substance and pure substance are synonyms. A sample of matter can be described as pure if the amount of impurities in the sample is negligible. Download a worksheet on Physical Properties of Matter for students to complete, and find additional teacher support from NSTA SciLinks. L2 L1 Gifted and ... Chapter 2Properties of Matter Section 2.3 Chemical Properties - Quia 1. Is the following sentence true or false? The substances in paraffin do not change when a candle burns. 2. Circle the letters of the compounds formed when a candle burns. a.paraffin b.hydrogen c.water d.carbon 3. What is a chemical property? 4. Is the following sentence true or false? Flammability is a material's ability to burn PDF Unit C1 - Revision Lesson 2 Rocks - Edexcel 2.1Describe that igneous rocks, such as granite, are: a) formed by the solidification of magma and lava, b) made of crystals whose size depends on the rate of cooling. 2.2Describe chalk and limestone as examples of sedimentary rocks. 2.3Describe how sedimentary rocks are formed by the compaction of layers of sediment over a very long time period.
Chapter 2 - Ms. Nichols - Google Section folders have the Powerpoint lesson notes, Lesson Practice homework, and the answer key to check your homework. Other folders may contain miscellaneous assignments or reviews. Selection File type icon File name Description Size Revision Time ... Extra Credit worksheet for Chapter 2 ... Education for Ministry | School of Theology | University of the South Education for Ministry. Education for Ministry (EfM) is a unique four-year distance learning certificate program in theological education based upon small-group study and practice. BibMe: Free Bibliography & Citation Maker - MLA, APA, Chicago, … BibMe Free Bibliography & Citation Maker - MLA, APA, Chicago, Harvard Chapter 2 Aluminum and copper have some properties in common, but there are differences besides their distinctive colors. • Aluminum is highly reflective and is often used in silver paint. • Pure copper can scratch the surface of aluminum because copper is harder than aluminum. • Copper is a conductor of heat or electric current.
PDF The Chemical Basis of Life CHaPTeR - Pearson Education 2.3 atoms consist of protons, neutrons, and electrons element have the same unique number of protons. This number is the element's atomic number. Thus, an atom of helium, with 2 protons, has an atomic number of 2. Unless otherwise indicated, an atom has an equal number of protons and electrons, and thus its net electrical charge is 0 (zero).
(PDF) Chapter 2 Atoms, Molecules, and Ions 2.1 Multiple-Choice ... Chapter 2 Atoms, Molecules, and Ions 2. Full file at
Experience Chemistry - Savvas (formerly Pearson K12 Learning) Storyline 2: Understanding Chemical Reactions. Investigation 4: Physical Properties of Materials. Experience 4.1 States of Matter. Experience 4.2 Modeling Phase Changes. Experience 4.3 Comparing Ionic and Molecular Compounds. Experience 4.4 Comparing Metals and Nonmetals. Experience 4.5 Water and Aqueous Systems.
PDF 2.3 Elements and Compounds - Henry County Schools Distinguishing Elements and Compounds Substances can be classified as elements or compounds. An elementis the simplest form of matter that has a unique set of properties. Oxygen and hydrogen are two of the more than 100 known elements. A compoundis a substance that contains two or more elements chemically combined in a fixed proportion.
PDF The Chemistry of Life SAMPLE ANSWER: Organisms use carbon compounds to form four types of molecules: lipids, carbohydrates, nucleic acids, and proteins. SAMPLE ANSWER: A lot of what that happens in an organism is based on chemical reactions. SAMPLE ANSWER: Enzymes are proteins that speed up chemical reactions that take place in cells.
Chapter 2 elements or compounds. •A colorless gas and a black solid appear. •Before heating, there was one substance. •After heating, there were two substances. •The blue-green solid must be a compound. •Based on the information given, it isn't possible to know if the colorless gas and the black solid are elements or compounds. Sample Problem 2.2
Chemistry (12th Edition) Chapter 2 - Matter and Change - 2.3 Elements ... Chapter 2 - Matter and Change - 2.3 Elements and Compounds - 2.3 Lesson check - Page 47: 26 Answer Electrolysis and burning can separate a compound into its substances. For example, electrolysis can separate water into hydrogen and oxygen. Work Step by Step Electrolysis and burning can separate a compound into its substances.
Chemistry (12th Edition) Chapter 2 - Matter and Change - 2 ... - GradeSaver Chemistry (12th Edition) answers to Chapter 2 - Matter and Change - 2.1 Properties of Matter - 2.1 Lesson Check - Page 37 1 including work step by step written by community members like you. Textbook Authors: Wilbraham, ISBN-10: 0132525763, ISBN-13: 978--13252-576-3, Publisher: Prentice Hall ... 2.3 Elements and Compounds - 2.3 Lesson check; 2 ...
PDF 2.3 Elements and Compounds - Weebly Title: chapter02_section03.ppt Author: Nicolette Kimball Created Date: 8/25/2014 7:37:04 PM
PDF SECTION 2.1 PROPERTIES OF MATTER (pages 39-42) SECTION 2.3 ELEMENTS AND COMPOUNDS (pages 48-52) This section explains a key difference between an element and a compound, and describes how chemicalsymbols and formulas are used to represent elements and compounds. It also summarizes the process for classifying substances and mixtures. Distinguishing Elements and Compounds (pages 48-49) 1.
PDF Chapter 2 Atoms, Molecules, and Ions - University of North Georgia compound, the numbers of atoms of each of its elements are always present in the same ratio (Figure 2.3). Figure 2.3 Copper(II) oxide, a powdery, black compound, results from the combination of two types of atoms—copper (brown spheres) and oxygen (red spheres)—in a 1:1 ratio. (credit: modification of work by "Chemicalinterest"/Wikimedia ...
PDF Sample Exercise 1.1 Distinguishing Among Elements, Compounds, and Mixtures volume of the box, using the correct number of significant figures in your answer. It takes 10.5 s for a sprinter to run 100.00 m. Calculate the average speed of the sprinter in meters per second, and express the result to the correct number of significant figures. Answer: 9.52 m/s (three significant figures) Practice Exercise
D0796287_BIO_C02_L03_Lesson_Review_Workbook_B (2).doc Then answer the questions. 1. Look at the diagram of the general structure of an amino acid. Color the amino group green. 2. Color the carboxyl group blue. 3. Color the R group red. 4. Color the same groups in the amino acids alanine and serine. 5. How many oxygen atoms are found in the carboxyl group?
PDF Pearson Chemistry 11 New South Wales Skills and Assessment Book WORKSHEET 2.3 Marvellous moles—the chemist's unit of measurement 61 WORKSHEET 2.4 Stoichiometry 1—mass- mass calculations 62 WORKSHEET 2.5 Molarity—measuring moles in solution 63 WORKSHEET 2.6 Gases—the ideal gas equation 64 WORKSHEET 2.7 Stoichiometry 2—mass-volume calculations 66
PDF The Chemical Context of Life - Pearson Education Key ConCepts 2.1 Matter consists of chemical elements in pure form and in combinations called compounds 2.2 An element's properties depend on the structure of its atoms 2.3 The formation and function of molecules depend on chemical bonding between atoms 2.4 Chemical reactions make and break chemical bonds
Pipeline Rules of Thumb Handbook 5E - Academia.edu This complete revision of Applied Process Design for Chemical and Petrochemical Plants, Volume 1 builds upon Ernest E. Ludwig's classic text to further enhance its use as a chemical engineering process design manual of methods and proven fundamentals.
PDF Pearson Queensland Chemistry 11 Skills and Assessment Book WORKSHEET 2.2.3 Molarity—measuring moles in solution 162 WORKSHEET 2.2.4 Solving solubility—predicting precipitation reactions 163 WORKSHEET 2.2.5 Concentration and strength— picturing acids and bases 164 WORKSHEET 2.2.6Reactions of acids— predicting products 165 WORKSHEET 2.2.7 Literacy review—full and ionic chemical equations 166
PDF Chapter 2 The Chemical Basis of Life - Los Angeles Mission College 2.3 Elements can combine to form compounds Compound—a substance consisting of two or more different elements combined in a fixed ratio -There are many compounds that consist of only two elements - Table salt (sodium chloride or NaCl) is an example - Sodium is a metal, and chloride is a poisonous gas
Chemistry 2.3: Elements and Compounds Flashcards | Quizlet element the simplest form of matter that has a unique set of properties. ex. oxygen, hydrogen compound a substance that contains two or more elements chemically combined in a fixed proportion. ex. sucrose is a compound made of carbon, oxygen, and hydrogen. has fixed composition (same chemical formula) chemical change


































0 Response to "45 2.3 elements and compounds worksheet answers pearson"
Post a Comment