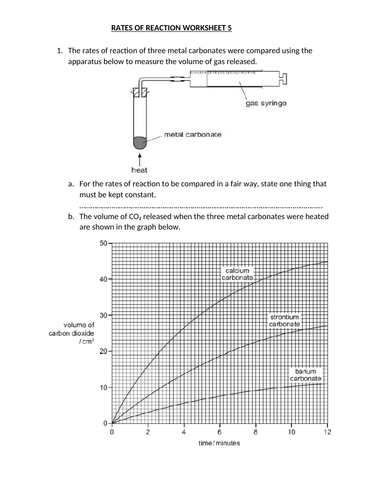
39 rates of reaction worksheet
Rates of Reaction worksheet.docx - Free download as Word Doc (.doc / .docx), PDF File (.pdf), Text File (.txt) or read online for free. Worksheet: Reaction Rates ... 2. Reaction rate refers to how quickly or slowly the ... 3. If a reaction is to occur, reacting particles must first ...
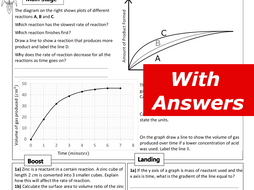
This worksheet gives you practice at calculating the rate of a reaction from data showing the quantity of product formed or the quantity of reactant used up ...8 pages
Rates of reaction worksheet
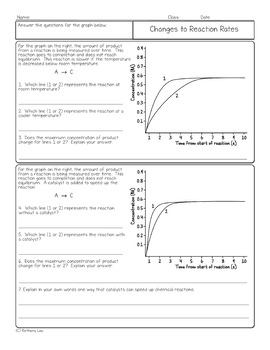
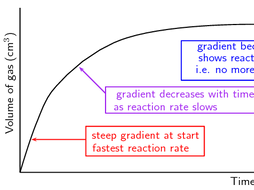
10.09.2020 · Note: Instantaneous rates are also known as differential rates. Thus for the reaction whose progress is plotted here, the actual rate (as measured by the increasing concentration of product) varies continuously, being greatest at time zero. The instantaneous rate of a reaction is given by the slope of a tangent to the concentration-vs.-time curve. There is another big idea for rates of reaction called collision theory. The collision theory says that as more collisions in a system occur, there will be more combinations of molecules bouncing into each other. If you have more possible combinations there is a higher chance that the molecules will complete the reaction. The reaction will happen faster which means the rate of … Topic 7 - Rates of reaction and energy changes. Rates of reaction. 7.4 Explain the effects on rates of reaction of changes in temperature, concentration, surface area to volume ratio of a solid and pressure (on reactions involving gases) in terms of frequency and/or energy of collisions between particles; OCR Chemistry B: 21st century. C8 ...
Rates of reaction worksheet. 20.01.2020 · The reaction of methane and steam produces carbon dioxide and water. _____ 10. Ammonia produced through the Haber process could … Worksheet on rates of reaction key words, graphs and definitions. I have used other people's resources for one of the questions, thanks to them. Rating: 4,6 · 46 reviews · Free · In stock Worksheet 1-1 - Measuring Reaction Rates. 1. A chemist wishes to determine the rate of reaction of zinc with hydrochloric acid. The.12 pages 12.2 Factors Affecting Reaction Rates. 12.3 Rate Laws. 12.4 Integrated Rate Laws. 12.5 Collision Theory. 12.6 Reaction Mechanisms. 12.7 Catalysis. Chapter 13. Fundamental Equilibrium Concepts. Introduction . 13.1 Chemical Equilibria. 13.2 Equilibrium Constants. 13.3 Shifting Equilibria: Le Châtelier’s Principle. 13.4 Equilibrium Calculations. Chapter 14. Acid-Base …
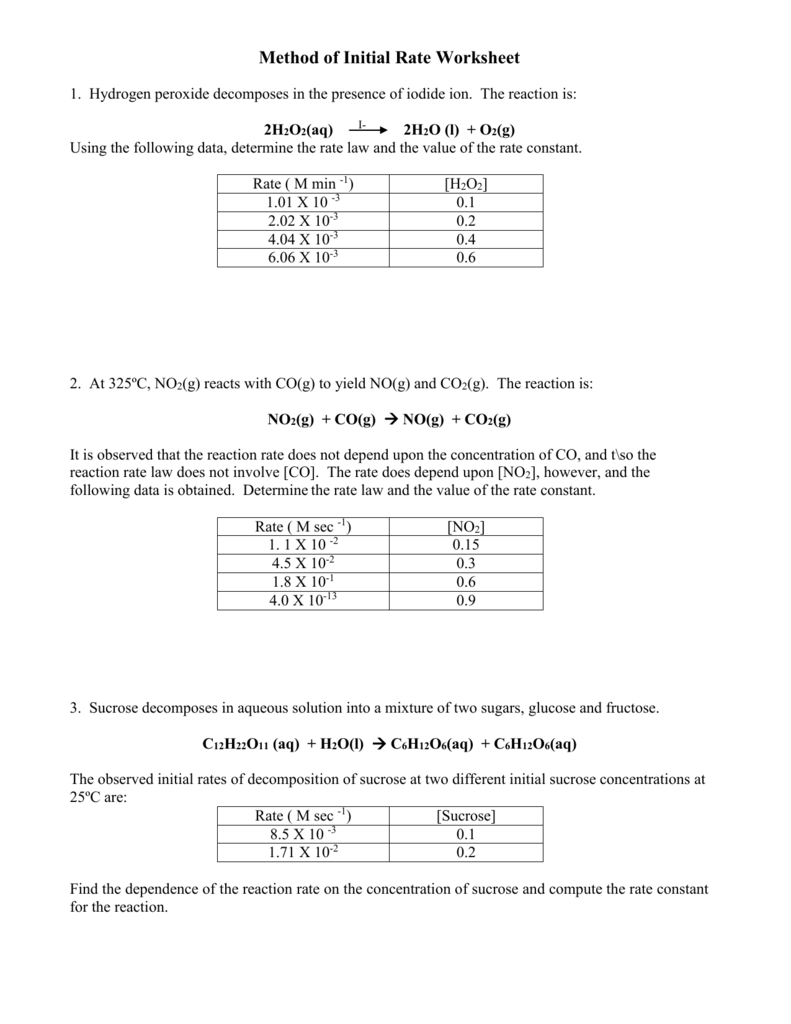
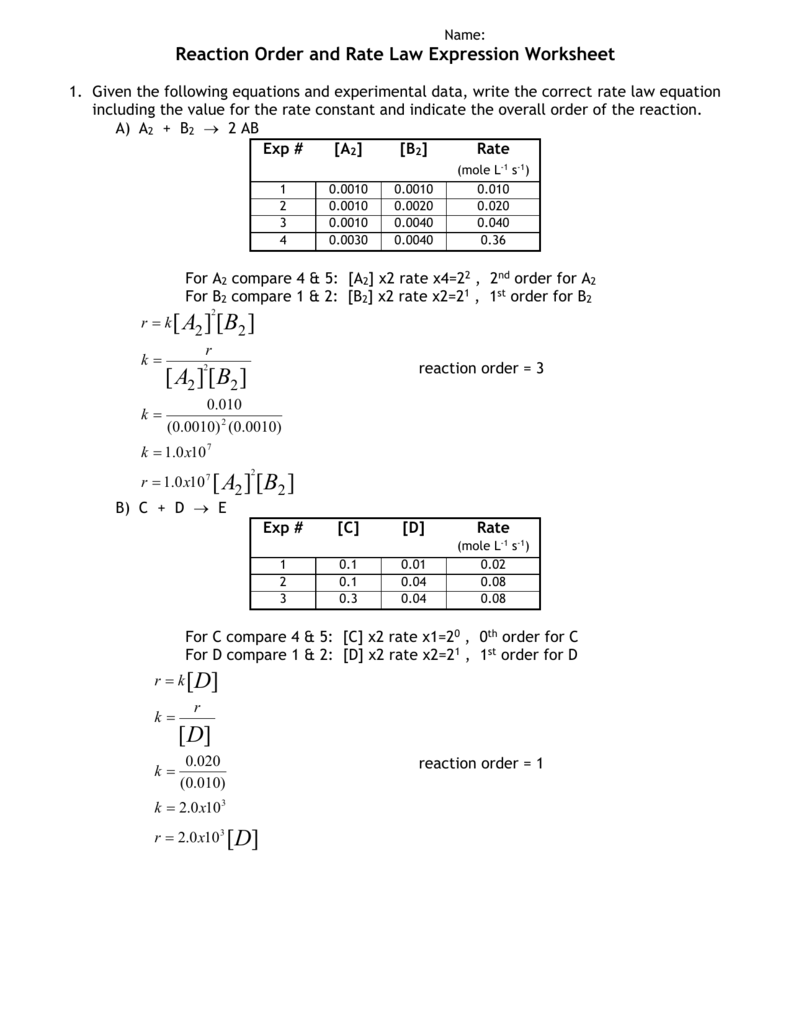
Introduction to reaction rates. Rate law and reaction order. Worked example: Determining a rate law using initial rates data. First-order reaction (with calculus) Plotting data for a first-order reaction. Half-life of a first-order reaction. Worked example: Using the first-order integrated rate law and half-life equations . Second-order reaction (with calculus) Half-life of a second-order ... b. the reaction is not at equilibrium, and will make more products at the expense of reactants. c. the reaction is not at equilibrium, and will make more reactants at the expense of products. d. the value of K will decrease until it is equal to Q. 9. If the reaction quotient Q has a larger value than the related equilibrium constant, K, _____ a ... Explore more than 1434 'Rate Of Reaction' resources for teachers, parents and pupils as well as related ... Chemical Reactions Worksheet - KS3 Chemistry. Indeed, solvolysis rates for substituted allylic halides are increased by both α and γ substitution. Friedel-Craft crotylation (CH ... Since the benzoin condensation is reversible, the 1,4-addition product is favored. This is known as the Stetter reaction; an example is shown below. C 6 H 5 C(OH)(CN) (–) + CH 2 =CHCN ——> C 6 H 5 COCH 2 CH 2 CN: For general use as an acyl …
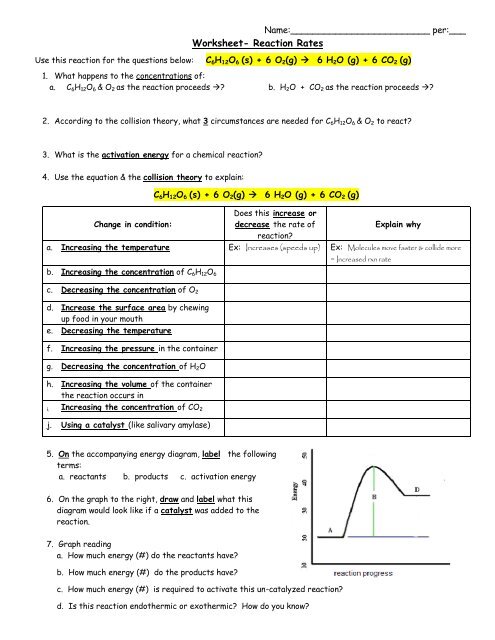
22.10.2020 · Download the student worksheet as MS Word or pdf. ... Knowledge of the application of this reaction to the manufacture of margarine by catalytic hydrogenation of unsaturated vegetable… 22 ii. halogens to produce dihalogenoalkanes ; 22 iv. steam, in the presence of an acid catalyst, to produce alcohols; GCSE. AQA Chemistry. 4.2 Bonding, … The reaction of CO with Cl 2 to form COCl 2 is another single-step reaction. A vessel is filled with only CO and Cl 2. Describe how equilibrium is achieved and the connection between the reaction rates and the equilibrium constant. Balanced Reaction: connection between the reaction rates and the equilibrium constant. CO (g)+ Cl 2 (g) COCl 2 (g ... To summarise, the rate of reaction can be affected by: (1) temperature, (2) particle size, and (3) concentration of the reactants. Still another factor which ...4 pages Worksheet- Reaction Rates ... C6H12O6 & O2 as the reaction proceeds →? ... What is the activation energy for a chemical reaction?2 pages
In this worksheet, we will practice describing the rate of a chemical reaction and explaining the effect the type of reagent and the surface area have on ...
Topic 7 - Rates of reaction and energy changes. Rates of reaction. 7.4 Explain the effects on rates of reaction of changes in temperature, concentration, surface area to volume ratio of a solid and pressure (on reactions involving gases) in terms of frequency and/or energy of collisions between particles; OCR Chemistry B: 21st century. C8 ...
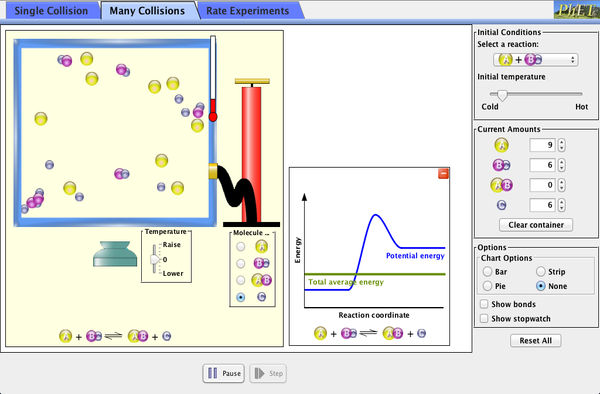
There is another big idea for rates of reaction called collision theory. The collision theory says that as more collisions in a system occur, there will be more combinations of molecules bouncing into each other. If you have more possible combinations there is a higher chance that the molecules will complete the reaction. The reaction will happen faster which means the rate of …
10.09.2020 · Note: Instantaneous rates are also known as differential rates. Thus for the reaction whose progress is plotted here, the actual rate (as measured by the increasing concentration of product) varies continuously, being greatest at time zero. The instantaneous rate of a reaction is given by the slope of a tangent to the concentration-vs.-time curve.







![Rate of a Chemical Reaction [Worksheet & Online Lesson] | TpT](https://ecdn.teacherspayteachers.com/thumbitem/Rate-of-a-Chemical-Reaction-Worksheet-Online-Lesson--5253135-1581793713/original-5253135-2.jpg)












0 Response to "39 rates of reaction worksheet"
Post a Comment