42 chemistry atomic number and mass number worksheet answers
Atomic Mass Formula The number of protons determines the identity of the atom, and isotopes have identical atomic numbers, so the atoms are of the same element. To find the atomic mass of chlorine, the atomic mass of each isotope is multiplied by the relative abundance (the percent abundance in decimal form)... Chemistry Atomic Number And Mass Number Worksheet Answer Details: Chemistry Worksheet, Atomic Number and Mass Number Goal: Atoms are composed of electrons, protons, and neutrons. It is the difference in the numbers of protons in the atoms that determine the different elements. You can determine the composition of an atom of any element from...
AQA 9-1 GCSE Chemistry 1 Paper 1 separate science past exam... In an atom, the number of electrons is equal to the number of protons in the nucleus. Atoms of different elements have different numbers of protons. You should be able to use the 1.1.5 Size and mass of atoms (AQA GCSE Chemistry 1, paper 1, Topic 1 "Atomic structure and the periodic table").

Chemistry atomic number and mass number worksheet answers
What is the difference between atomic number and mass number? Atomic Number vs Mass Number Atoms are characterized by their atomic numbers and mass numbers. In the periodic table, atoms are arranged Mass number of an element is more related with the mass of it. However, it is not giving the exact mass of the atom. There are some elements... Chemistry Atomic Number And Mass Number Worksheets Answer... Work Power And Energy Worksheets Answers. Types Of Chemical Bonds Worksheets Answer Key. Isotopes & Relative Atomic Mass (solutions, examples, videos) Chemistry: How to calculate Atomic Mass of an element, Isotopes, Isotope Notation, Atomic Mass Unit (amu), Relative atomic mass, How to The extra neutrons just change the mass of the atom and its density. Some of the atoms of certain isotopes are unstable because of the extra number of...
Chemistry atomic number and mass number worksheet answers. 49 Balancing Chemical Equations Worksheets [with Answers] Download our Balancing Chemical Equations Worksheets to learn more about the topic. 4 Balancing Equations Worksheets with Answers. 5 What are Different Types of Chemical It is necessary to balance it because there must be equal number of atoms on both the sides of the... Difference Between Atomic Mass and Mass Number The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a decimal number. Atomic mass value sometimes change over time in publications as scientists revise the natural isotope abundance of elements. Atomic number, atomic mass, and isotopes (article) | Khan Academy Fundamental properties of atoms including atomic number and atomic mass. The atomic number is the number of protons in an atom, and isotopes have the same atomic number but differ in the number of neutrons. Atomic Number and Mass Number | Difference Between Atomic... The atomic number is defined as the total number of protons or electrons in an element. Hence atomic number is useful in differentiating one element from another. Normally, atomic mass and mass numbers are two different terms and may differ slightly. In most cases, they are not the same.
Periodic Table with Atomic Mass | Science Notes and Projects Chemistry Problems With Answers. The atomic mass is the average number of protons and neutrons in atoms of a chemical elements, allowing for The IUPAC actually recommends a range of atomic mass values for most elements because the isotopic ratio isn't a constant everywhere on Earth. PDF Name Block Atomic Structure Worksheet Label the parts (Explain your answer fully. ) If an atom has 35 protons in the nucleus, how many electrons will it have orbiting the nucleus? What is the atomic number of the atom in the diagram above? How many neutrons are in the nucleus of an atom with an atomic number of 25? (use Periodic Table for mass). Atomic Number And Atomic Mass Number : Definition, Examples... Atomic Number of element depends only on the number of protons in the elements. it does not depends on number of electrons or neutron. Question 2 An ion with atomic mass number 37 possess 1 unit of -ve charge. If the ion contains 11.1% mole neutrons than electrons. GCSE Science Revision Chemistry "Atomic Number and Mass..." 9-1 GCSE Chemistry Paper 1 Atomic Structure and the Periodic Table.
Atomic Mass Worksheets With Answers, Jobs EcityWorks Atomic Mass Atomic Number Worksheet 1 November 6 chemistry atomic number and mass number worksheet answers, atomic mass number Ecityworks will offer the trendies Atomic Mass Worksheets With Answers jobs at the New job tool on the homepage.Let's check our site regularly to... PDF Basic Atomic Structure Worksheet The 3 particles of the atom The atomic number gives the "identity" of an element as well as its location on the periodic table. No two different elements will have the. atomic number. of an element is the average mass of an element's naturally Occurring atom, or _ of each isotope. Determine the number of atomic number, mass number, proton... Periodic table online worksheet for 8. You can do the exercises online or download the worksheet as in terms of charge. b. determine the number of protons, neutrons and electrons in an atom; and c. locate the atomic number and mass number in the What do you want to do? Check my answers. The Parts of the Periodic Table | Atomic Numbers The atomic number is the number of protons in the nucleus of an atom. In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass — at that time, the nucleus had not yet been discovered, and there was no understanding at all...
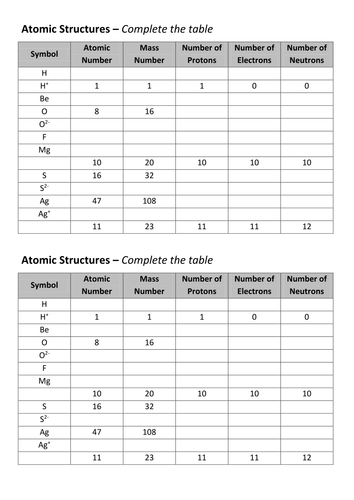
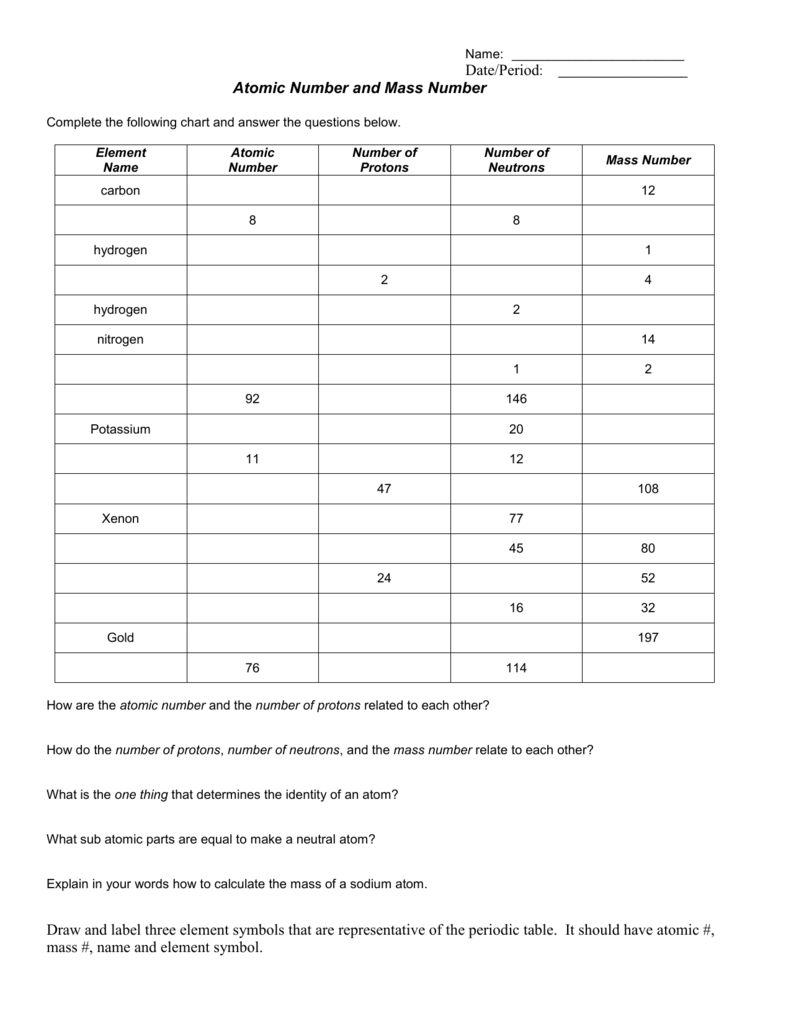
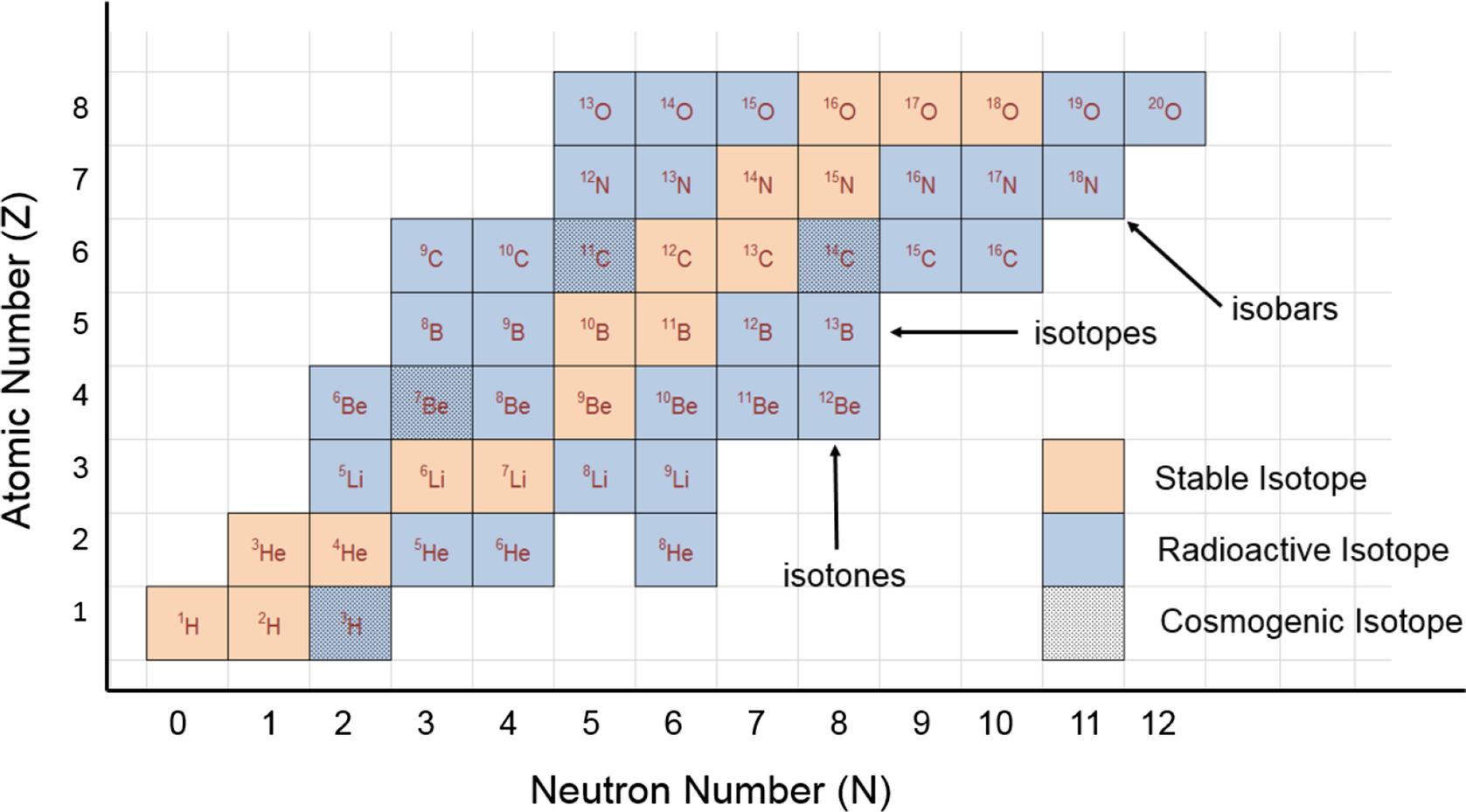
Mass number - atomic number = # neutrons Mass number: total number of protons and neutrons in the nucleus (not listed on the periodic table, since it varies). Atoms of the same element have the same atomic number, but may have different mass numbers. Isotopic notation for a particular atom (also called nuclide symbol notation)
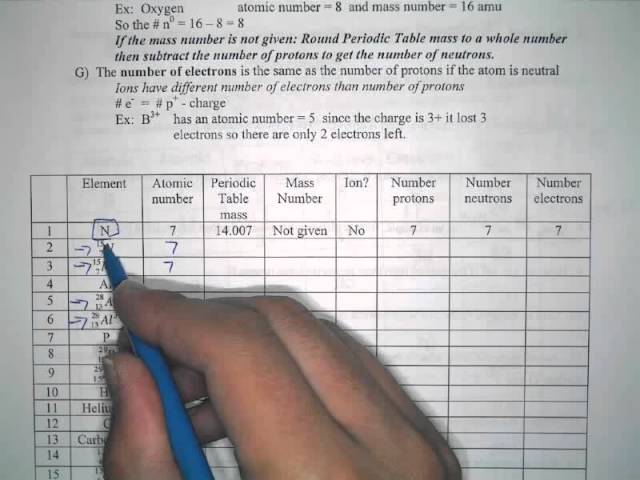
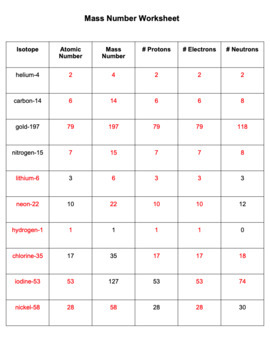
Chemistry Atomic Number And Mass Number Worksheet Answer... Atomic mass and atomic number answers displaying top 8 worksheets found for this concept. Atomic mass and atomic number worksheet key name of element symbol atomic number atomic mass protons neutrons electrons copper cu 29 64 29 35 29 tin sn 50 119 50 69 50 iodine i 53 127 53...
NCERT Solutions for Class 9 Science Chapter 4 Structure of Atom Atomic Number and Mass Number. These solutions are part of NCERT Solutions for Class 9 Science. Answer. Isotopes: Atoms of same element having same atomic number but different mass number. Questions from NCERT Text Book.
Website's listing atomic number atomic mass worksheet... Chemistry Worksheet, Atomic Number and Mass Number Goal: Atoms are composed of electrons, protons, and neutrons. It is the difference in the numbers of protons in the atoms that determine the different elements. Atomic number and atomic mass worksheet. Answers included.
Atomic Structure Worksheets An awesome collection of free atomic structure worksheets for teachers. An atom is the smallest We will advance on to looking further into the nucleus and explore nuclear chemistry of atoms that The number of protons is the atomic number and the mass number is the sum of the protons and...
PDF Chemistry of Matter | ATOMIC NUMBER Subtract the atomic number from the atomic mass. 11. Use your knowledge of atomic calculations to complete the chart. 16. Answer the questions below based on the elements in question #15. (1) Which elements had a filled outermost shell? He & Ne (2) Which element would be...
3 Ways to Calculate Atomic Mass - wikiHow The atomic mass is the number of grams of the element in one mole of atoms of the element. This is a very useful property when it comes to practical Atomic masses, when expressed in amu, as on the periodic table, are technically unitless. However, by simply multiplying an atomic mass by 1 g/mol, a...
Questions and Answers - How do I find the number of protons... The atomic number is the number located in the upper left corner and the atomic weight is the Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton Happily, to find the mass number, all you need to do is round the atomic weight to the nearest whole...
Atomic number and mass number - Atomic structure - AQA - GCSE... The number of subatomic particles in an atom can be calculated from the atom's atomic number and mass number. An atom contains equal numbers of protons and electrons. Since protons and electrons have equal and opposite charges, this means that atoms are have no overall electrical...
Atomic Number and Mass Number | Introduction to Chemistry Atomic number, chemical symbol, and mass numberCarbon has an atomic number of six, and two stable isotopes with mass numbers of twelve and thirteen, respectively. Its average atomic mass is 12.11. Scientists determine the atomic mass by calculating the mean of the mass numbers for its...
Questions by Topic - 1.1 Atomic Structure - AQA Chemistry A-level... Home › Chemistry Revision › AQA A-Level › Physical Chemistry I › Atomic Structure Questions. Mass Number and Isotopes 2 MS.
Isotopes & Relative Atomic Mass (solutions, examples, videos) Chemistry: How to calculate Atomic Mass of an element, Isotopes, Isotope Notation, Atomic Mass Unit (amu), Relative atomic mass, How to The extra neutrons just change the mass of the atom and its density. Some of the atoms of certain isotopes are unstable because of the extra number of...
Chemistry Atomic Number And Mass Number Worksheets Answer... Work Power And Energy Worksheets Answers. Types Of Chemical Bonds Worksheets Answer Key.
What is the difference between atomic number and mass number? Atomic Number vs Mass Number Atoms are characterized by their atomic numbers and mass numbers. In the periodic table, atoms are arranged Mass number of an element is more related with the mass of it. However, it is not giving the exact mass of the atom. There are some elements...

/element-list-names-atomic-numbers-606529_V1_FINAL-f332cfc84a494b7782d84fc986cdaf86.png)








![Atomic Number, Mass Number and Isotopes [Worksheet]](https://ecdn.teacherspayteachers.com/thumbitem/Atomic-Number-Mass-Number-and-Isotopes-Worksheet--2519547-1645560933/original-2519547-4.jpg)












0 Response to "42 chemistry atomic number and mass number worksheet answers"
Post a Comment