42 specific heat capacity worksheet answers
Specific Heat Capacity Worksheet With Answers Sep 28, 2021 · Specific heat capacity worksheet with answers. A piece of copper with a mass of 218 g has heat capacity of 83 9 j c what is the specific heat capacity of copper. View answer the mass of a metal that appears to be gold is 4 30 g. Identify each variables by name the units associated with it. DOC Specific Heat Worksheet - Socorro Independent School District Specific Heat Worksheet Specific Heat Worksheet Name (in ink): C = q/m∆T, where q = heat energy, m = mass, and T = temperature Remember, ∆T = (Tfinal - Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1.
ANSWER KEY - west-windsor-plainsboro.k12.nj.us Hall of fame question- depends on your answers for 1-5: A brilliant chemistry student harnesses all of the energy released from problems 1, 2, and 4 to heat up an unknown sample with 1000 times the mass of water in problem 3. She raises the temperature from 50 C to the same final temperature as problem 5.

Specific heat capacity worksheet answers
DOC HEAT Practice Problems - Murrieta Valley Unified School ... ANSWER KEY. HEAT Practice Problems . Q = m x ∆T x C . 5.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092 cal/g °C) 27.6 cal. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°C to 29°C? (Specific heat ... sch4u-specific heat and heat capacity worksheet with answers Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24.5oC to 26.4oC. How much heat did this sample absorb? c for water = 4.18 J/goC (ans. 2.66 kJ) 2. How much heat in kilojoules has to be removed from 225g of water to lower its temperature from 25.0oC to 10.0oC? (ans. –14.1 kJ) 3. PDF Chemistry Specific Heat Worksheet Answers Chemistry specific heat worksheet answers. Answers included on separate sheet. For q m c δ t. Also includes a spreadsheet to show how the calculations have been done. Use the data in the table to answer the following questions. Specific heat capacity handout answer key specific heat practice work. Explain how they differ from each other.
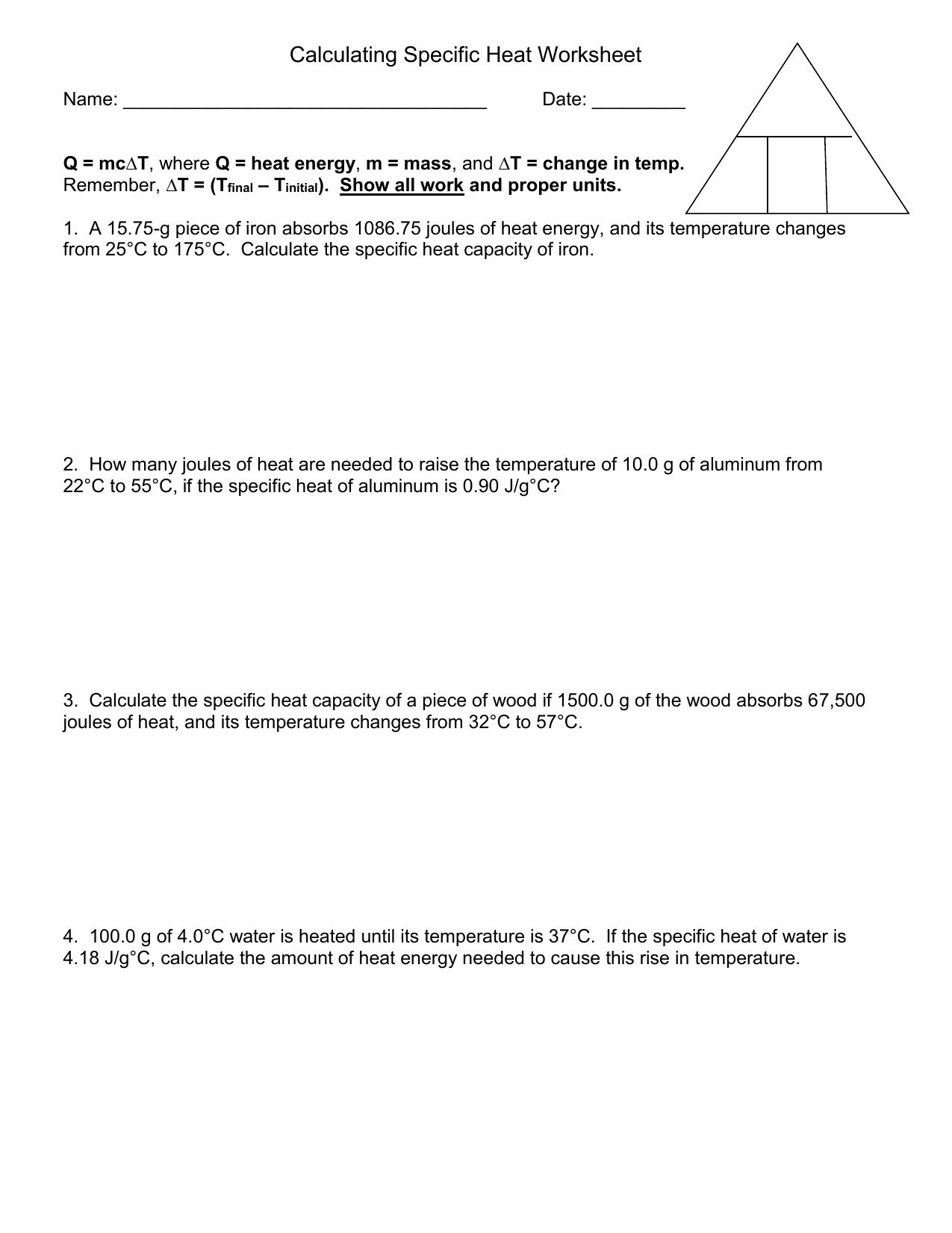
Specific heat capacity worksheet answers. PDF North St. Paul-Maplewood Oakdale / Overview 1 2 3 4 5. 6 7. 8 Name Specific Heat Worksheet Cp = q/mAT, where q = heat energy, m = mass, and T = temperature A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 250C to 1750C. Worksheet- Introduction to Specific Heat Capacities Water has the highest specific heat capacity and metal has the lowest. 6. Here are the heat capacities of the four substances: 0.10 cal/g °c, 0.25 cal/g °c, 1.0 cal/g °c, & 0.2 cal/g °c. Match & then label each substance with its specific heat capacity on the graph. See graph above. 7. If something has a high specific heat capacity will it ... Specific Heat Capacity Worksheet With Answers - Universal ... Nov 15, 2021 · Specific heat capacity worksheet with answers. Answers are provided at the end of the worksheet without units. Also includes a spreadsheet to show how the calculations have been done. For q m oc a t. A 15 75 g piece of iron sorbs 1086 75 joules of heat energy and its temperature changes from 25 0 1750c. Specific Heat Problems Worksheet - Studying Worksheets Specific Heat Worksheet Answers. Answers on thermal properties of matter MCQ questions PDF covers topics. The specific heat capacity of a material is the internal energy change when 100 kg of that material changes by 100 K.
Specific Heat of Water & Metals: Physics Lab - Video ... 2021-11-01 · The specific heat, or more fully, the specific heat capacity, is a measure of how much energy it takes to increase the temperature of 1 kilogram of a substance by 1 degree Celsius (or Kelvin). For ... PDF Worksheet- Calculations involving Specific Heat Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. Specific And Latent Heat Worksheet Answers - Studying ... Specific Heat Worksheet Answers. How much water at 32C is needed to just melt 15 kg of ice at -10C. Here are the heat capacities of the four substances. How much water at 50 C is needed to just melt 22 kg of ice. Specific Heat Worksheet Name in ink. Its specific latent heat of fusion is 241 kJkg. Specific heat worksheet name in ink. PDF Specific Heat Wksht20130116145212867 Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C.
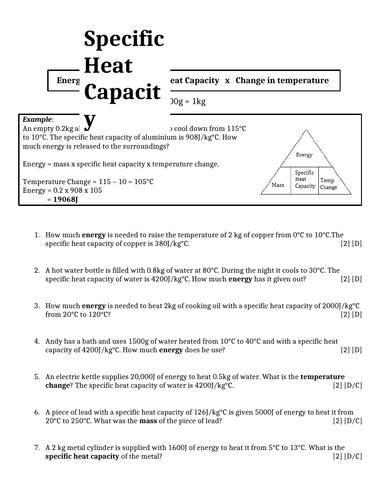
Specific Heat Capacity Worksheet (with answers) | Teaching ... Jan 21, 2015 · docx, 19.93 KB. xlsx, 17.87 KB. Two page worksheet using Specific Heat Capacity. Questions start easy then become gradually harder. Answers included on separate sheet. Also includes a spreadsheet to show how the calculations have been done. PDF Specific Heat Calculations Worksheet With Answers Calculating Specific Heat Worksheet Answers | akademiexcel.com Specific Heat and Heat Capacity Worksheet DIRECTIONS: Use q = (m)(Cp))(ΔT) to solve the following problems. Show all work and units. Ex: How many joules of heat are needed to raise the temperature of 10.0 g of Honors Chemistry Worksheet - Specific Heat How much heat energy produced this change in temperature? (Ans. 2,000 J) 2. When 300. cal of energy is lost from a 125 g object, the temperature decreases from 45.0°C to 40.0°C. What is the specific heat of this object? (Ans. 0.48 cal/g oC or 2.0 J/g oC) 3. 1,200 cal of heat energy is added to a liquid with a specific heat of 0.57 cal/g°C. Factoring Trinomials Worksheet Answers With Work - Explore ... Algebra worksheets factoring trinomials. Factoring trinomials a 1 write each trinomial in factored form as the product of two binomials. 25 scaffolded questions that start relatively easy and end with some real challenges. The child may be encouraged to work out the problem on a piece of paper before entering the solution.
Specific Heat Capacity - Worksheet (Key) | PDF ... A 15.75-9 piece of iron absorbs L}86.75joules of heat energy, and its temperature changes from 25"C to 175"C. Calculate the specific heat capacity of iron. h= lf ,7f gz o,otSZfb Q- ^, A T cx 4/o J/4'c
PDF Worksheet Introduction To Specific Heat Capacities Answers Answers 20T Specific Heat worksheetCalorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry Introduction to Specific Heat Capacity (SHC) Heat Capacity, Specific Heat, and Calorimetry intro to specific heatIntroduction to Page 6/37
Specific Heat Capacity 1 - GCSE Physics Worksheet Answers ... This video explains the answers to the first Specific Heat Capacity GCSE Physics Worksheet. These worksheets are very useful for revising important GCSE Phys...
Quiz & Worksheet - Calculating Specific Heat Capacity ... About This Quiz & Worksheet. This quiz and worksheet gauge your knowledge of specific heat capacity and how it is calculated. You will be quizzed on terms, such as …
Specific Heat Capacity 2 - GCSE Physics Worksheet Answers ... This video explains the answers to the second Specific Heat Capacity GCSE Physics Worksheet. These worksheets are very useful for revising important GCSE Phy...
Specific Heat Worksheet With Answers - Askworksheet Specific heat worksheet with answers. A 15 75 g piece of iron sorbs 1086 75 joules of heat energy and its temperature changes from 25 0 1750c. Heat is not the same as temperature yet they are related. Use q m δt cp to solve the following problems. Show all work and proper units. Perform calculations usin 1. Gold has a specific heat of 0 129 j gx0c.
PDF Specific Heat Calculations Worksheet With Answers Get Free Specific Heat Calculations Worksheet With Answers its temperature changes from 25°C to 175°C. Calculate the specific heat capacity of iron.
Specific Heat Worksheet With Answers - Thekidsworksheet Specific heat worksheet with answers. Show all work and proper units. Heat is not the same as temperature yet they are related. Calculate the specific heat capacity of iron. Identify each variables by name the units associated with it. Specific heat calculations worksheet answers specific heat worksheet name in ink.
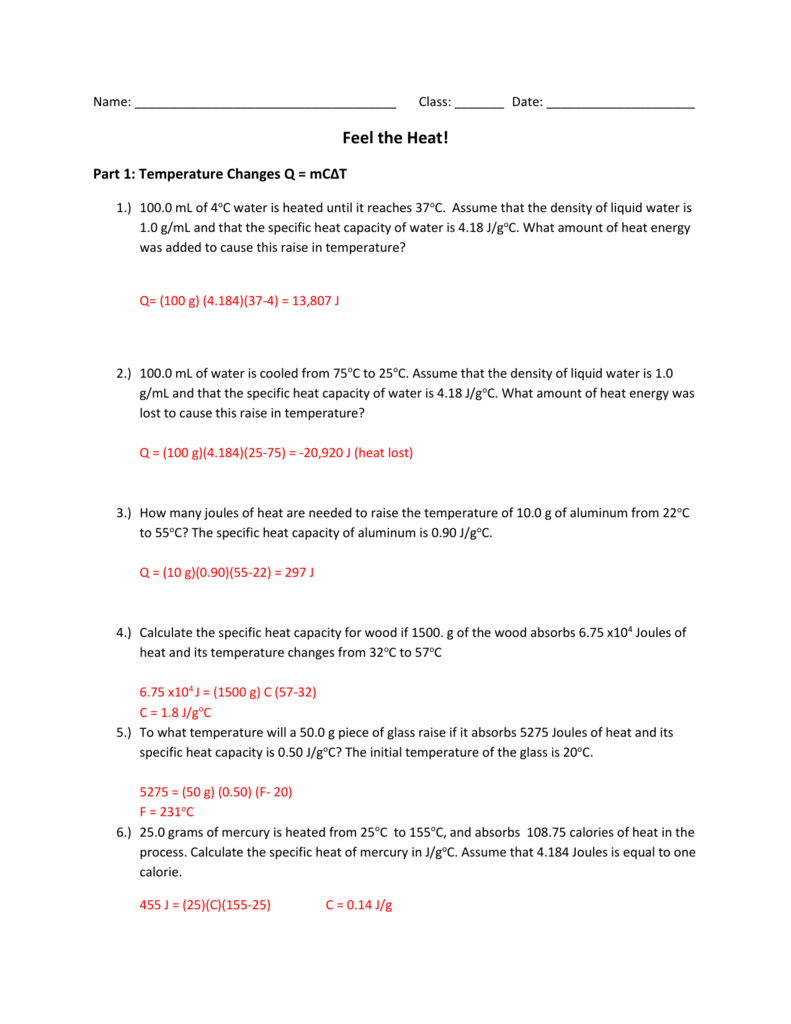
PDF Specific Heat and Heat Capacity Worksheet Specific Heat and Heat Capacity Worksheet DIRECTIONS: Use q = (m)(Cp))(ΔT) to solve the following problems. Show all work and units. Ex: How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°C to 55°C, if the specific heat of aluminum is 0.90 J/g°C? 1.
PDF Latent heat and Specific heat capacity questions. Latent heat and Specific heat capacity questions. 1. How much water at 50°C is needed to just melt 2.2 kg of ice at 0°C? 2. How much water at 32°C is needed to just melt 1.5 kg of ice at -10°C? 3. How much steam at 100° is needed to just melt 5 kg of ice at -15°C? 4. A copper cup holds some cold water at 4°C.
PDF Chemistry Specific Heat Worksheet Answers Chemistry specific heat worksheet answers. Answers included on separate sheet. For q m c δ t. Also includes a spreadsheet to show how the calculations have been done. Use the data in the table to answer the following questions. Specific heat capacity handout answer key specific heat practice work. Explain how they differ from each other.
sch4u-specific heat and heat capacity worksheet with answers Specific Heat and Heat Capacity Worksheet 1 The temperature of 335 g of water changed from 24.5oC to 26.4oC. How much heat did this sample absorb? c for water = 4.18 J/goC (ans. 2.66 kJ) 2. How much heat in kilojoules has to be removed from 225g of water to lower its temperature from 25.0oC to 10.0oC? (ans. –14.1 kJ) 3.
DOC HEAT Practice Problems - Murrieta Valley Unified School ... ANSWER KEY. HEAT Practice Problems . Q = m x ∆T x C . 5.0 g of copper was heated from 20°C to 80°C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092 cal/g °C) 27.6 cal. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°C to 29°C? (Specific heat ...



































0 Response to "42 specific heat capacity worksheet answers"
Post a Comment